Hibiscrub
BRAILLE TEXT:
HIBISCRUB
Font: Marburg Medium 28,5 pt. EU Braille
• • • • • • •
H I B
• • • • • • • • • • • • • •• •
SCRUB
58mm
0HfBTSCRUB®
4% w/v Cutaneous Solution Chlorhexidine gluconate 40 mg/ml
Height-85mm Height-85mm Height-85mm Height-85mm Height-85mm
|
What Hibiscrub looks like and contents of the pack Hibiscrub is a clear, red, slightly viscous solution and comes in white bottles containing 250 ml, 500 ml or 5 litres of liquid. Marketing Authorisation Holder Regent Medical (Overseas) Limited, Medlock Street, Oldham, Lancashire, OL1 3HS, UK. Manufacturer BCM Ltd., 1 Thane Road West, Nottingham, NG2 3AA, UK. This leaflet was last revised in 03/2016. | |||||||
Base Label 58mm
bottle after “EXP”. The expiry date refers to the last day of that month. Do not store above 25°C. Store in the original package.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Hibiscrub contains
• The active substance is chlorhexidine gluconate 4% w/v (40 mg/ml)
• The other ingredients are poloxamer 237, lauryl dimethyl, isopropyl alcohol, amine oxide, glycerol, macrogol 7 glycerol cocoate, gluconolactone, perfume (Herbacol), ponceau 4R (E124), sodium hydroxide and purified water.
•HIBISCRUB®
4% w/v Cutaneous Solution Chlorhexidine gluconate 40 mg/ml
Antimicrobial Skin Cleanser for antiseptic handwashing on the ward, pre-operative surgical hand disinfection and patient pre-and post-operative skin antisepsis.
Read the package leaflet before use, which is given when the label is peeled back.
Excipients: poloxamer 237, isopropyl alcohol, lauryl dimethyl amine oxide, glycerol, macrogol 7 glycerol cocoate, gluconolactone, perfume (Herbacol), ponceau 4R (E124), sodium hydroxide and purified water.
Precautions: Avoid contact with eyes, brain, meninges, eyes and middle ear. Use with care in newborn babies, especially those born prematurely. Hibiscrub may cause chemical skin burns.
FOR EXTERNAL USE ONLY
Keep out of the sight and reach of children. Do not store above 25°C. Store in the original package.
Regent Medical (Overseas) Limited, Medlock Street, Oldham, Lancashire,
OL1 3HS, UK.
PA 1218/1/1;
PL 22099/0001
Hibiscrub® is a trade mark of Regent Medical Ltd.
O
z
o
Q
|
1 | ||||||
|
1 | ||||||
|
Package leaflet: Information for the patient • HIBISCRUB® 4% w/v Cutaneous Solution Chlorhexidine gluconate Read all of this leaflet carefully before you start using this medicine because it contains important information for you. Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you. • Keep this leaflet. You may need to read it again. • Ask your pharmacist if you need more information or advice. • If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any |
Ireland HPRA Pharmacovigilance Earlsfort Terrace IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie e-mail: medsafety@hpra.ie United Kingdom Yellow Card Scheme Website: By reporting side effects you can help provide more information on the safety of this medicine. 5. How to store Hibiscrub Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is printed on the | |||||
|
; | ||||||
J097 930784 > V
5 "060097
Page 16-47mm Front Cover - 55mm
Inside Cover
Page 15
Hibiscrub cutaneous solution contains the active ingredient chlorhexidine gluconate 4% w/v (40 mg/ml) which has antimicrobial properties.
Hibiscrub is used to disinfect the hands and skin prior to or after an operation or clean procedure (pre- and post-operative hand and skin disinfection / antisepsis).
Hibiscrub is also used to disinfect hands after handling infected materials (antiseptic handwashing).
2. What you need to know before you use Hibiscrub
Do not use Hibiscrub
• if you are allergic (hypersensitive) to chlorhexidine
data suggests these are very rare (probably affect fewer than 1 in every 10,000 people).
• Serious allergic reactions which can cause breathing difficulties, weakness, collapse and death, and can sometimes be preceded by swelling (in particular lip/tongue and facial swelling), or rash.
Allergic reactions to chlorhexidine-impregnated patches have been reported rarely when used in newborn babies.
At the first sign of any of these reactions, tell your doctor, pharmacist or nurse and application of Hibiscrub should be stopped immediately.
Other possible side effects, for which it is not known how often they occur, are: allergic skin disorders such as dermatitis (inflammation of the skin), pruritus (itch), erythema (redness of the skin), eczema, rash, urticaria (hives), skin irritation and blisters.
Inflammation of the membranes of the brain and spinal cord has been reported when they have come into direct contact with chlorhexidine.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via:
possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Hibiscrub is and what it is used for
2. What you need to know before you use Hibiscrub
3. How to use Hibiscrub
4. Possible side effects
5. How to store Hibiscrub
6. Contents of the pack and other information
1. What Hibiscrub is and what it is used for
Hibiscrub belongs to a group of medicines called antiseptics. This means that it helps to prevent infections by killing germs on the skin.
Page 4-47mm Page 13-47mm
Page 14
Page 3
If you accidentally swallow any Hibiscrub
You should go to your nearest Accident and Emergency department or contact your doctor immediately. Take this leaflet and any other packaging with you so they know what you have taken.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur; it is not known how often they occur but available
gluconate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or
nurse before using Hibiscrub.
• Avoid contact with the eyes, brain, meninges (the membranes surrounding the brain and spinal cord) and middle ear.
If Hibiscrub comes into contact with the eyes, wash out immediately and thoroughly with water.
• In patients with head or spinal injuries or a perforated ear drum (an ear drum with a hole or tear in it), the benefit of use in pre-operative preparation should be evaluated against the risk of contact.
• Common bleaches (which contain hypochlorites) may cause brown stains to develop on fabrics which have previously been in contact with chlorhexidine.
• Hibiscrub's action may be reduced if used with soap or detergents.
• If you go into hospital, let your doctor know if you are using Hibiscrub.
Children
Use with care in newborn babies, especially those born prematurely. Hibiscrub may cause chemical skin burns.
3. How to use Hibiscrub
Always use this medicine exactly as described in this leaflet or as
• Wash the whole body. Start with the face. Work down the body. Pay special attention to the skin around the nose, under the arms, around the belly button, between the legs and around the bottom.
• Rinse the whole body.
• Repeat the wash. Use the same amount of Hibiscrub. This time, wash the hair as well.
• Rinse the whole body and dry thoroughly with a clean towel.
For Preventing Infection After Your Operation
Wash the whole of your body, excluding the operation site, in the bath or the shower. This is usually done on the third day after the operation. Use the method described above.
Page 12-47mm Page 5-47mm
Page 6
Page 11
|
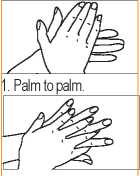
1. Palm to palm. | |
|
2. Right palm over left dorsum and left palm over right dorsum | |
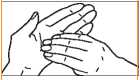
3. Palm to palm fingers interlaced.
5. Rotational rubbing of right thumb clasping in left palm and vice versa.
6. Rotational rubbing backwards and forwards of tips of fingers and thumb of right hand in left palm and vice versa.
4. Back of fingers to opposing palms with fingers interlocked.
Continue washing hands and wrists until one minute has elapsed. Clean the fingernails with a brush or scraper. Rinse thoroughly. For pre-operative use repeat the wash a second time for two minutes.
|
1 | ||||||
|
1 | ||||||
|
The Hibiscrub handwashing technique is based on a procedure described by Ayliffe GAJ et al. J Clin Path 1978; 31: 923. For Antiseptic Handwashing on the Ward Wet the hands and forearms, apply 5 ml of Hibiscrub and wash for one minute. Rinse thoroughly and dry. For Preventing Infection Before Your Operation Wash the whole of your body in the bath or the shower on at least two occasions. This is usually done on the day before the operation and the day of the operation. Use the following method: • Use 25 ml of Hibiscrub as a soap solution. |
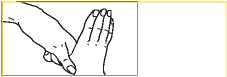
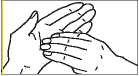
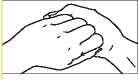
your doctor, pharmacist or nurse have told you. Check with your doctor, pharmacist or nurse if you are not sure. FOR EXTERNAL USE ONLY Do not inject. Do not use in the body's internal spaces. Chlorhexidine must not be used in direct contact with nerve tissue, for example brain and spinal cord tissue. For Pre-operative Surgical Hand Disinfection Wet the hands and forearms. Wash with 5 ml of Hibiscrub using the following procedure, each step consisting of five strokes backwards and forwards. | |||||
|
i | ||||||
HIBISCRUB
Font: Marburg Medium 28,5 pt. EU Braille
• • • • • • •
H I B
• • • • • • • • • • • • • •• •
SCRUB
45mm
pHiBiSCRuB®
4% w/v Cutaneous Solution
Chlorhexidine gluconate 40 mg/ml
• •
• •
115mm 115mm 115mm 115mm 115mm
igi
a
T
n white bottles containing 250 ml, 500 ml or 5 litres of iquid.
Marketing
Authorisation
Holder
Regent Medical (Overseas) Limited Wedlock Street, Oldham, Lancashire, OL1 3HS, UK.
Manufacturer
BCM Ltd., 1 Thane Road West, Nottingham,
NG2 3AA, UK.
This leaflet was last revised in 03/2016.
Hibiscrub® is a trade mark of Regent Medical Ltd.
Base Label - 45mm
6. Contents of the pack and other information
What Hibiscrub contains
- The active ingredient is chlorhexidine gluconate 4% w/v (40 mg/ml)
- The other ingredients are poloxamer 237, isopropyl alcohol, lauryl dimethyl amine oxide, glycerol, macrogol 7 glycerol cocoate, gluconolactone, perfume (Herbacol), ponceau 4R (E124), sodium hydroxide and purified water.
What Hibiscrub looks like and contents of the pack
Hibiscrub is a clear, red, slightly viscous solution and comes
Antimicrobial Skin Cleanser for antiseptic handwashing on the ward, pre-operative surgical hand disinfection and patient pre- anc post-operative skin antisepsis.
Read the package leaflet before use, which is given when the label is peeled back.
Excipients: poloxamer 237, isopropyl alcohol, lauryl dimethyl amine oxide, glycerol, macrogol 7 glycerol cocoate, gluconolactone, perfume (Herbacol), ponceau 4R (E124), sodium hydroxide and purified water.
Precautions: Avoid contact with eyes, brain, meninges, eyes and middle ear.
Use with care in newborn babies, especially those born prematurely. Hibiscrub may cause chemical skin burns.
FOR EXTERNAL USE ONLY
Keep out of the sight and reach of children. Do not store above 25°C. Store in the original package.
Regent Medical (Overseas) Limited, Medlock Street, Oldham, Lancashire, OL1 3HS, UK.
PA 1218/1/1; PL 22099/0001 Hibiscrub® is a trade mark of Regent Medical Ltd.
O
SD
tr
O
060097
tbc
930746
Package leaflet: nformation for the catient
0 HIBISCRUB®
4% w/v Cutaneous Solution Chlorhexidine gluconate
Read all of this leaflet carefully before you start
using this medicine
because it contains important information for you.
Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you. -Keep this leaflet. You may need to read it again.
-Ask your pharmacist if you need more information or advice.
-If you get any side effects, talk to your doctor, pharmacist
5. How to store Hibiscrub
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is printed on the bottle after "EXP". The expiry date refers to the last day of that month.
Do not store above 25°C. Store in the original package.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no onger use. These measures will help protect the environment.
Page 16 - 32mm Front Cover - 42mm
Inside Cover - 42mm
Page 15 - 32mm
infections by killing germs on the skin.
Hibiscrub is used to disinfect the hands and skin prior to or after an operation or a clean procedure (pre- and postoperative hand and skin disinfection/ antisepsis).
Hibiscrub is can also be used to disinfect hands after handling infected materials (antiseptic handwashing).
2. What you need to know before you use Hibiscrub
Do not use
Hibiscrub if • you are allergic (hypersensitive) to chlorhexidine gluconate or any of the other ingredients of this medicine (listed in section 6).
Allergic reactions to chlorhexidine-impreg nated patches have been reported rarely when used in newborn babies.
At the first sign of any of these reactions, tell your doctor, pharmacist or nurse and application of Hibiscrub should be stopped immediately.
Other possible side effects, for which it is not known how often they occur, are: allergic skin disorders such as dermatitis
(inflammation of the skin), pruritus (itch), erythema (redness of the skin), eczema, rash, urticaria (hives), skin irritation and blisters.
Inflammation of the membranes of the brain and spinal cord has been reported when they have come
into direct contact with chlorhexidine.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via:
Ireland
HPRA
Pharmacovigilance Earlsfort Terrace IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Wfebsite: www.hpra.ie e-mail:
United Kingdom
Yellow Card Scheme Website:
wcard
By reporting side effects you can help provide more information on the safety of this medicine
or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Hibiscrub is and what it is used for
2. What you need to know before you use Hibiscrub
3. How to use Hibiscrub
4. Possible side effects
5. How to store Hibiscrub
6. Contents of the pack and other information
1. What Hibiscrub is and what it is used for
Hibiscrub belongs to a group of medicines called antiseptics. This means that it helps to prevent
Page 4 - 32mm
Page 13 - 32mm
Page 14-32mm : Page 3-32mm
doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur; it is not known how often they occur but available data suggests these are very rare (probably affect fewer than 1 in every 10,000 people).
• Serious allergic reactions which can cause breathing difficulties, weakness, collapse and death, and can sometimes be preceded by swelling (in particular lip/tongue and facial swelling), or rash.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Hibiscrub.
• Avoid contact with the eyes, brain, meninges (the membranes surrounding the brain and spinal cord) and middle ear. If Hibiscrub comes into contact with the eyes, wash out immediately and thoroughly with water.
• In patients with head or spinal injuries or a perforated ear drum (an ear drum with a hole or tear in it), the benefit of use in pre-operative preparation should be evaluated against the risk of contact.
• Common bleaches (which contain hypochlorites) may cause brown stains to develop on
fabrics, which have previously been in contact with chlorhexidine.
• Hibiscrub’s action may be reduced if used with soap or detergents.
• If you go into hospital, let your doctor know if you are using Hibiscrub.
Children
Use with care in newborn babies, especially those born prematurely. Hibiscrub may cause chemical skin burns.
3. How to use Hibiscrub
Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you. Check with your doctor, pharmacist or nurse if you are not sure.
thoroughly with a clean towel.
For Preventing Infection After Your Operation
Wash the whole of your body, excluding the operation site, in the bath or the shower. This is usually done on the third day after the operation. Use the method described above.
If you accidentally swallow any Hibiscrub
You should go to your nearest Accident and Emergency department or contact your doctor immediately. Take this leaflet and any other packaging with you so they know what you have taken. If you have any further questions on the use of this medicine, ask your
Page 12 - 32mm
Page 5 -32mm
Page 6 - 32mm Page 11 - 32mm
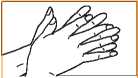
3. Palm to palm fingers nterlaced.
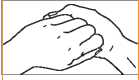
4. Back of fingers to opposing palms with ingers interlocked.
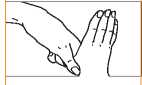
5. Rotational rubbing of right thumb clasping in left palm and vice versa.
6. Rotational rubbing backwards and forwards of tips of fingers and thumb of right hand in left palm and vice versa.
Continue washing hands and wrists until one minute has elapsed.
Clean the fingernails with a brush or scraper. Rinse thoroughly. For pre-operative use repeat the wash a second time for two minutes.
The Hibiscrub handwashing technique is based on a procedure described by Ayliffe GAJ et al. J Clin Path 1978; 31: 923.
For Antiseptic Handwashing on the ward
Wet the hands and forearms, apply 5 ml of Hibiscrub and wash for one minute. Rinse thoroughly and dry.
For Preventing Infection Before Your Operation
Wash the whole of
your body in the bath or the shower on at least two occasions. This is usually done on the day before the operation and the day of the operation. Use the following method:
• Use 25 ml of Hibiscrub as a soap solution.
• Wash the whole body. Start with the face. Work down the body. Pay special attention to the skin around the nose, under the arms, around the belly button, between the legs and around the bottom.
• Rinse the whole body.
• Repeat the wash. Use the same amount of Hibiscrub. This time, wash the hair as well.
• Rinse the whole body and dry
FOR EXTERNAL USE ONLY
Do not inject. Do not use in the body’s internal spaces.
Chlorhexidine must not be used in direct contact with nerve tissue, for example brain and spinal cord tissue.
Pre-operative Surgical Hand Disinfection
Wet the hands and forearms. Wash with 5 ml of Hibiscrub using the following procedure, each step consisting of five strokes backwards and forwards.
2. Right palm over left dorsum and left palm over right dorsum.
HIBISCRUB
Font: Marburg Medium 28,5 pt. EU Braille
• •• • •••• • • • ••••• •• •
• •• •
HIBISCRUB
# HIBISCRUB®
4% w/v Cutaneous Solution Chlorhexidine gluconate 40 mg/ml
• •• • •••• • •
• ••••• •• • • • • •
102mm
Height - 150 mm Height - 150 mm Height - 150mm
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is printed on the bottle after “EXP”. The expiry date refers to the last day of that month.
Do not store above 25°C. Store in the original package.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Hibiscrub contains
• The active substance is chlorhexidine gluconate 4% w/v (40 mg/ml)
• The other ingredients are poloxamer 237, lauryl dimethyl, isopropyl alcohol, amine oxide, glycerol, macrogol 7 glycerol cocoate, gluconolactone, perfume (Herbacol), ponceau 4R (E124), sodium hydroxide and purified water.
What Hibiscrub looks like and contents of the pack
Hibiscrub is a clear, red, slightly viscous solution and comes in white bottles containing 250 ml, 500 ml or 5 litres of liquid.
Marketing Authorisation Holder
Regent Medical (Overseas) Limited, Medlock Street, Oldham, Lancashire, OL1 3HS, UK.
BCM Ltd., 1 Thane Road West, Nottingham, NG2 3AA, UK. This leaflet was last revised in 03/2016.
Base Label - 102mm
£ a6ed
J0AOQ 0P|SU|
sq) sjo/sq Aep sq) uo auop A||ensn s| s|qj_ suojseooo om))sbs| )e uo JSMoqs sq) jo q)eq sq) u| Apoq jnoA )0 0|oqM sq) qse/\/\ uoqejedo JnoA ejojeg uoyosjui BuyusASJd joj
Ajp pue A|q6nojoq) ssujy spujuj 0uo jo) qsBM pus qmos!q!H )o |uu g A|dde ‘suubsjo) pus spueq sq))s/\/\ Pjb/\a sq) uo BmqseMpueH opdsspuv joj
ZZ6 ■ te I8Z6I- q)Bd UNO r |e P TVQ Aq paquosop 0jnp0oojd b uo p0SBq s| snbpqos) BujqseMpueq qnjospiH sqj_
ssyiuiiu omj jo) 0iu|} puooos b qsBM sqpesdsj 0sn SA!)ejsdo-sjd joy A|q6nojoq) 0su|y jsdejosjo qsmq b qyM snewsBui) sq) ub0|o pssde|s ssq 0jnu|uj 0uo ||)un sjsum pus spueq SujqsBM snu|)uoo
BSJ0A 0OjA pue UJ|Bd )J0| hi puei| )ljBu jo q ill nip
PUB SJsBujJ JO sdj) JO BSJ0A 0O|A PUB UJ|Bd D0| p0>|OO|J0)U! SJsBujJ
spjB/vuoj pus spjB/v\>)OBq uj 6ujdse|o qumq) )q6u q)|m sw|ed Bujsoddo
Buiqqm jeuojjejoy 9 jo Buiqqm jeuojjejoy g o) sjoBmj jo >peg p
|
P8OB|J0}UI sj06uij tu|ed oj tuiBd £ |
lunsjop )l|6u J8A0 tu|Bd }j0| pus tunsjop l]0| J8A0 tu|Bd z |
LU|Bd 0} tUIBd ' \, | ||
SpjBMJOJ PUB SpjBA/V>|OBq SS>|OJ)S sal) jo Bujjsisuoo ds)s qoB0 ‘0jnp0oojd 6u!MO||oj sq) Bujsn qmos!q!H jo |iu g qyM qse/\/\ siujesjoj pus spusq sq))s/\/\ uoyosjuisia pubh |BO|Bjns SAyejsdo-sJd joj
snss|) pjoo |BU|ds pus upjq 0|dujBX0 joj ‘snssj) 0AJ0U qyM jobjuoo }O0J|p u| posn 0q )ou )sniu sujpjxsqjoiqo
ssoeds |BUJ0}U| s.Apoq sq) u| 0sn )ou oq 'psfuj )ou oq
A1NO 3Sn 1VNd31X3 tdOd
■0jns )ou 0jb noA y osjnu jo ppeiujeqd ‘jopop jnoA q}|M>|O0qo noA p|0) 0ABq osjnu jo ppeiujeqd ‘jopop jnoA sb jo )S|jes| s|q) u| psquossp sb A|pexs supipsiu s|q) ssn sAbmiv
sujnq up|s |eo|UJsqo ssneo Abuj qmosiquq A|SjnjBUJSjd ujoq ssoqj A||Bpsdss ‘ssjqeq ujoqMSU u| sjbo qyM ssn
qmos!q!H
Bupn sjb noA y mou>| jopop jnoA )S| ‘|eydsoq o)U| 06 noA y.
sjusBjsjsp
jo dsos qyM pssn y psonpsj sq Abuj uoyoe s,qnjos|q|H.
sujpjxsqjoiqo qyMpepoo u| ussq ApnojASjd SAeq qojqM souqej uo do|SAsp oj supjs UMOjq ssnso Abuj (ssjuoiqoodAq ujBiuoo qopM) ssqoBsp uoujujoq .
qoBjuoo ^o
>|su sqjisujBBB psjBn|BAS sq ppoqs uojjBJBdsjd SAjjBJsdo-sjd u| ssn p ii^susq sqj ‘(}| u| jbsj jo spq b qjjM ujrnp jbs ub) lump jbs psjBJopisd b jo ssunfuj |BU|ds jo pssq qjjM sjusjjBd u|.
■jsjbm qjjM A|q6nojoqj pus A|S}B!psiuuJ! jno qsBM ‘ssAs sqj qjjM jobjuoo ojuj ssiuoo qnjos!q||-| jbs s|pp|iu pus (pjoo |BU|ds pus upjq sqj Bujpunojjns ssuBjqiusiu sqj) ssBupsiu ‘upjq ‘ssAs sqj qjiMjOBjuoo ppAy • qmos!q!H Bupn sjo^sq ssjnu jojspBiujBqd ‘jopop jnoA 0}>||bj_
(g uojjoss u| ps}S||) supipsiu sppo sjusjpsjBu! jsqjo sqjp Aub jo sjBuoon|6 sujpjxsqjoiqo oj (sAmsussjsdAq) oiBjsub sjb noA i!.
(BupsBMpuBq oijdssijuB) s|bus}biu psps^uj Bujipusq jsys spusq psppip oj pssn osp s| qmosjqip
(SISdSSIJUB / UOIJOSppip U|>|S pus pueq SAjjBJsdo-jsod pue -sjd) sjnpsoojd uesp jo uojjBJsdo ue jsye jo oj joud u|>|s pus spusq squos^upp oj pssn s| qnjospip
ssiysdojd iBiqojoiiuijuE ssq qopM (|iu/6iu otr) a/m sjBUOon|6 sujpjxsqjoiqo jusipsjBui saijob sqjsujBiuoo uo|}n|os snosusjno qnjosjqiy
Uj>|S
sqj uo siujsB 6u!||!>| Aq suoijos^ui jusASjd oj sd|sq y jsq} sussiu s|qj_ soijdssiiuB ps||BO ssupipsiu ^o dnojB s oj s6uo|sq qmosjqip
joj pssn s| y jeqAA pue s; qnjosiqiH }eq/\A y
uoijeiujo^u! jsqjo pus >|OBd sqj yo sjusjuoo g qnjosjqiH sjojs oj MO|q S spsys spp s|q|ssod p qnjosiqiH ssn oj moh S qnjosiqiH ssn noA sjopq mou>| oj pssu noA }eq/v\ z JOi pssn s| y jsqM pus s| qnjosiqnq }eq/v\ ■ j, is lies | sim ui s| }eq/\/|
p UOjJOSS SSS }9|iBS| s|qj u| ps}Sj| }ou spsys sp|s siqpsod Aub sspn|ou| s|qx ssjnu jo jspsujjBqd ‘jopop jnoA oj >||B} ‘spsys spp Aub jsB noA i| • sojAps jo uojjBUjjoiU! sjoiu pssu noAy jspsujjBqd jnoA >|sv • uibBb y pssj oj pssu Abuj nox Plfesi s|qj dss>| • noA p|oj SAsq ssjnu jo jspsujjBqd ‘jopop jnoA sb jo }S|iBS| s|qj u| psquossp sb A|pexs supipsiu s|qj ssn sAbmiv noA joi uopeuijoiui }UB}jodujj suiejuoo y ssneosq suioipsuj siqj Buisn jjbjs noA sjo^sq Aynisjeo jsyesi siqj io ye pesy
sjBUOon|6 su!p|xsqjo|qo uoynios snosuejno a/m %p
anaosiaiH •
operation and the day of the operation. Use the following method:
• Use 25 ml of Hibiscrub as a soap solution.
• Wash the whole body. Start with the face. Work down the body. Pay special attention to the skin around the nose, under the arms, around the belly button, between the legs and around the bottom.
• Rinse the whole body.
• Repeat the wash. Use the same amount of Hibiscrub. This time, wash the hair as well.
• Rinse the whole body and dry thoroughly with a clean towel.
For Preventing Infection After Your Operation
Wash the whole of your body, excluding the operation site, in the bath or the shower. This is usually done on the third day after the operation. Use the method described above.
If you accidentally swallow any Hibiscrub
You should go to your nearest Accident and Emergency department or contact your doctor immediately. Take this leaflet and any other packaging with you so they know what you have taken.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur; it is not known how often they occur but available data suggests these are very rare (probably affect fewer than 1 in every 10,000 people).
• Serious allergic reactions which can cause breathing difficulties, weakness, collapse and death, and can sometimes be preceded by swelling (in particular lip/tongue and facial swelling), or rash.
Allergic reactions to chlorhexidine-impregnated patches have been reported rarely when used in newborn babies.
At the first sign of any of these reactions, tell your doctor, pharmacist or nurse and application of Hibiscrub should be stopped immediately.
Other possible side effects, for which it is not known how often they occur, are: allergic skin disorders such as dermatitis (inflammation of the skin), pruritus (itch), erythema (redness of the skin), eczema, rash, urticaria (hives), skin irritation and blisters.
Inflammation of the membranes of the brain and spinal cord has been reported when they have come into direct contact with chlorhexidine.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via:
HPRA Pharmacovigilance Earlsfort Terrace IRL - Dublin 2 Tel: +353 1 6764971 Fax: +353 1 6762517 Website: www.hpra.ie e-mail: medsafety@hpra.ie
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
Antimicrobial skin cleanser for antiseptic handwashing on the ward, ore-operative surgical hand disinfection, and patient pre- and post-operative skin antisepsis.
Read the package leaflet before use, which is given when the label is peeled back.
Excipients: poloxamer 237, isopropyl alcohol, lauryl dimethyl amine oxide, glycerol, macrogol 7 glycerol cocoate, gluconolactone, perfume (Herbacol), ponceau 4R (E124), sodium hydroxide and purified water.
Precautions: Avoid contact with eyes, brain, meninges and middle ear. Use with care in newborn babies, especially those born prematurely. Hibiscrub may cause chemical skin burns. Keep out of the sight and reach of children.
Do not store above 25°C. Store in the original package.
Regent Medical (Overseas) Limited
Medlock Street, Oldham, Lancashire, OL1 3HS, UK
PA 1218/1/1; PL 22099/0001
Trade Mark of Regent Medical Overseas Ltd.
06
097
76

Page 5 - 91mm Page 6 - 91mm Front Cover - 99mm