Humulin I (Isophane) 100 Iu/Ml Suspension For Injection In Cartridge
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Humulin I (Isophane) 100 IU/ml suspension for injection in cartridge
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
1 ml contains 100 IU human insulin (produced in E. coli by recombinant DNA technology).
One cartridge contains 3 ml equivalent to 300 IU of isophane insulin.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
A suspension for injection in a cartridge.
Humulin I is a sterile suspension of a white, crystalline precipitate of isophane human insulin in an isotonic phosphate buffer.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
For the treatment of patients with diabetes mellitus who require insulin for the maintenance of glucose homeostasis.
Posology and method of administration
4.2
The dosage should be determined by the physician, according to the requirement of the patient.
Humulin I should be given by subcutaneous injection but may although not recommended, also be given by intramuscular injection. This formulation should not be administered intravenously.
Subcutaneous administration should be in the upper arms, thighs, buttocks or abdomen. Use of injection sites should be rotated so that the same site is not used more than approximately once a month.
Care should be taken when injecting any Humulin insulin preparations to ensure that a blood vessel has not been entered. After any insulin injection, the injection site should not be massaged. Patients must be educated to use proper injection techniques.
Each pack contains a patient information leaflet with instructions on how to inject insulin.
4.3 Contraindications
Hypoglycaemia.
Hypersensitivity to Humulin or to the formulation excipients, unless used as part of a desensitisation programme.
Under no circumstances should any Humulin formulation other than Humulin S (Soluble) be given intravenously.
4.4 Special warnings and precautions for use
Transferring a patient to another type or brand of insulin should be done under strict medical supervision. Changes in strength, brand (manufacturer), type (soluble, isophane, mixture), species (animal, human, human insulin analogue), and/or method of manufacture (recombinant DNA versus animal-source insulin) may result in the need for a change in dosage.
Some patients taking human insulin may require a change in dosage from that used with animal-source insulins. If an adjustment is needed, it may occur with the first dose or during the first several weeks or months.
A few patients who experienced hypoglycaemic reactions after transfer to human insulin have reported that the early warning symptoms were less pronounced or different from those experienced with their previous animal insulin. Patients whose blood glucose is greatly improved, e.g. by intensified insulin therapy, may lose some or all of the warning symptoms of hypoglycaemia and should be advised accordingly. Other conditions which may make the early warning symptoms of hypoglycaemia different or less pronounced include long duration of diabetes, diabetic nerve disease, or medications such as beta blockers. Uncorrected hypoglycaemic and hyperglycaemic reactions can cause loss of consciousness, coma or death.
The use of dosages which are inadequate or discontinuation of treatment, especially in insulin-dependent diabetics, may lead to hyperglycaemia and diabetic ketoacidosis; conditions which are potentially lethal.
Treatment with human insulin may cause formation of antibodies, but titres of antibodies are lower than those to purified animal insulin.
Insulin requirements may change significantly in diseases of the adrenal, pituitary or thyroid glands and in the presence of renal or hepatic impairment.
Insulin requirements may be increased during illness or emotional disturbances.
Adjustment of insulin dosage may also be necessary if patients change their level of physical activity or change their usual diet.
Combination of human insulin with pioglitazone
Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. This should be kept in mind, if treatment with the combination of pioglitazone and human insulin is considered. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued, if any deterioration in cardiac symptoms occurs.
4.5 Interactions with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with glucose metabolism and therefore the physician should be consulted when using other medications in addition to human insulin (see section 4.4). The physician must therefore take possible interactions into account and should always ask his patients about any medicinal products they take.
Insulin requirements may be increased by substances with hyperglycaemic activity, such as glucocorticoids, thyroid hormones, growth hormone, danazol, beta2- sympatomimetics (such as ritodrine, salbutamol, terbutaline), thiazides.
Insulin requirements may be reduced in the presence of substances with hypoglycaemic activity, such as oral hypoglycaemics (OHA), salicylates (for example, acetylsalicylic acid), certain antidepressants (monoamine oxidase inhibitors), certain angiotensin converting enzyme (ACE) inhibitors (captopril, enalapril), angiotensin II receptor blockers, non-selective betablocking agents and alcohol.
Somatostatin analogues (octreotide, lanreotide) may both decrease or increase insulin dose requirements.
4.6 Fertility, pregnancy and lactation
It is essential to maintain good control of the insulin treated (insulin-dependent or gestational diabetes) patient throughout pregnancy. Insulin requirements usually fall during the first trimester and increase during the second and third trimesters. Patients with diabetes should be advised to inform their doctors if they are pregnant or are contemplating pregnancy.
Careful monitoring of glucose control, as well as general health, is essential in pregnant patients with diabetes.
Patients with diabetes who are lactating may require adjustments in insulin dose and/or diet.
4.7 Effects on ability to drive and use machines
The patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia. This may constitute a risk in situations where these abilities are of special importance (e.g. driving a car or operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia whilst driving, this is particularly important in those who have reduced or absent awareness of the warning signs of hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be considered in these circumstances.
4.8 Undesirable effects
Hypoglycaemia is the most frequent undesirable effect of insulin therapy that a patient with diabetes may suffer. Severe hypoglycaemia may lead to loss of consciousness, and in extreme cases, death. No specific frequency for hypoglycaemia is presented, since hypoglycaemia is a result of both the insulin dose and other factors e.g. a patient's level of diet and exercise.
Local allergy in patients is common (1/100 to < 1/10). Redness, swelling, and itching can occur at the site of insulin injection. This condition usually resolves in a few days to a few weeks. In some instances, local reactions may be related to factors other than insulin, such as irritants in the skin cleansing agent or poor injection technique.
Systemic allergy, which is very rare (< 1/10,000) but potentially more serious, is a generalised allergy to insulin. It may cause rash over the whole body, shortness of breath, wheezing, reduction in blood pressure, fast pulse, or sweating. Severe cases of generalised allergy may be life-threatening.
In the rare event of a severe allergy to Humulin, treatment is required immediately. A change of insulin or desensitisation may be required.
Lipodystrophy at the injection site is uncommon (1/1,000 to < 1/100).
Cases of oedema have been reported with insulin therapy, particularly if previous poor metabolic control is improved by intensified insulin therapy.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the United Kingdom: Yellow Card Scheme, Website: www.mhra.gov.uk/yellowcard
4.9 Overdose
Insulin has no specific overdose definitions, because serum glucose concentrations are a result of complex interactions between insulin levels, glucose availability and other metabolic processes. Hypoglycaemia may occur as a result of an excess of insulin relative to food intake and energy expenditure.
Hypoglycaemia may be associated with listlessness, confusion, palpitations, headache, sweating and vomiting.
Mild hypoglycaemic episodes will respond to oral administration of glucose or sugar products.
Correction of moderately severe hypoglycaemia can be accomplished by intramuscular or subcutaneous administration of glucagon, followed by oral carbohydrate when the patient recovers sufficiently. Patients who fail to respond to glucagon must be given glucose solution intravenously.
If the patient is comatose, glucagon should be administered intramuscularly or subcutaneously. However, glucose solution must be given intravenously, if glucagon is not available or if the patient fails to respond to glucagon. The patient should be given a meal as soon as consciousness is recovered.
Sustained carbonhydrate intake and observation may be necessary because hypoglycaemia
may occur after apparent clinical recovery.
5 PHARMACOLOGICAL PROPERTIES
5.1
Pharmacodynamic properties
Pharmaco-therapeutic group: Humulin I: ATC code A10A C01. Humulin I is an intermediate acting insulin preparation.
The prime activity of insulin is the regulation of glucose metabolism.
In addition insulin has several anabolic and anti-catabolic actions on a variety of different tissues. Within muscle tissue this includes increasing glycogen, fatty acid, glycerol and protein synthesis and amino acid uptake, while decreasing glycogenolysis, gluconeogenesis, ketogenesis, lipolysis, protein catabolism and amino acid output.
The typical activity profile (glucose utilisation curve) following subcutaneous injection is illustrated below by the heavy line. Variations that a patient may experience in timing and/or intensity of insulin activity are illustrated by the shaded area. Individual variability will depend on factors such as size of dose, site of injection temperature and physical activity of the patient.
Humulin I
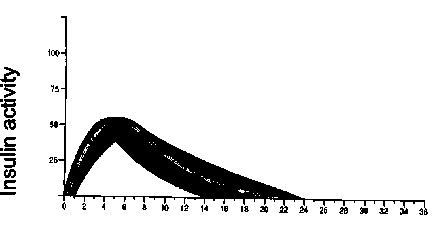
Time (hours)
5.2 Pharmacokinetic properties
The pharmacokinetics of insulin do not reflect the metabolic action of that hormone. Therefore, it is more appropriate to examine glucose utilisation curves (as discussed above) when considering the activity of insulin.
5.3 Preclinical safety data
Humulin is human insulin produced by recombinant technology. No serious events have been reported in subchronic toxicology studies. Human insulin was not mutagenic in a series of in vitro and in vivo genetic toxicity assays.
6.1 List of excipients
rn-cresol
glycerol
phenol
protamine sulfate
dibasic sodium phosphate 7H2O
zinc oxide
water for injections.
The following may be used to adjust pH; hydrochloric acid and/or sodium hydroxide.
6.2 Incompatibilities
Humulin preparations should not be mixed with insulins produced by other manufacturers or with animal insulin preparations.
6.3 Shelf life
Unused cartridge 3 years.
After cartridge insertion 28 days.
6.4 Special precautions for storage
Unused cartridge
Store in a refrigerator (2 °C - 8 °C). Do not freeze. Do not expose to excessive heat or direct sunlight.
After cartridge insertion
Store below 30°C. Do not refrigerate. The pen with the inserted cartridge should not be stored with the needle attached.
6.5 Nature and content of container
3 ml solution in a cartridge (type I glass) with a plunger head at the bottom (rubber) and disc seal at the top (rubber). Pack size of 5.
6.6 Special precautions for disposal and other handling
Do not reuse needles. Dispose of the needle in a responsible manner. Needles and pens must not be shared. Cartridges can be used until empty, then properly discard. Any unused product or waste material should be disposed of in accordance with local requirements.
Instructions for use and handling
A suspension for injection in a 3 ml cartridge to be used with a CE marked pen as recommended in the information provided by the device manufacturer.
a) Preparing a dose
Cartridges containing Humulin I formulation should be rolled in the palms of the hands ten times and inverted 1800 ten times immediately before use to resuspend the insulin until it appears uniform cloudy or milky. If not, repeat the above procedure until contents are mixed. Cartridges contain a small glass bead to assist mixing. Do not shake vigorously as this may cause frothing, which may interfere with the correct measurement of the dose.
The cartridges should be examined frequently and should not be used if clumps of material are present or if solid white particles stick to the bottom or wall of the cartridge, giving a frosted appearance.
The cartridges are not designed to allow any other insulin to be mixed in the cartridge.
Cartridges are not designed to be refilled.
The manufacturer's instructions with each individual pen must be followed for loading the cartridge, attaching the needle and administering the insulin injection.
b) Injecting a dose
Inject the correct dose of insulin, as directed by your doctor or diabetes specialist nurse.
Use of the injection sites should be rotated so that the same is not used more than approximately once a month.
Each pack contains a patient information leaflet with instructions on how to inject insulin.
7 MARKETING AUTHORISATION HOLDER
Eli Lilly and Company Limited, Lilly House, PriestleyRoad, Basingstoke, Hampshire RG24 9NL
Trading style: Lilly Industries Limited
8 MARKETING AUTHORISATION NUMBER(S)
PL 00006/0257
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
23 November 1990/ 24 April 2006
10 DATE OF REVISION OF THE TEXT
21/08/2015