Innohep 20 000 Iu/Ml


PATIENT INFORMATION LEAFLET 2506
16.07.15[4]
innohep® 20,000 lU/ml Syringe
(tinzaparin sodium)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section
4.
• In this leaflet innohep® 20,000 IU/ml Syringe will be called innohep®.
What is in this leaflet
1. What innohep® is and what it is used for
2. What you need to know before you use innohep®
3. How to use innohep®
4. Possible side effects
5. How to store innohep®
6. Contents of the pack and other information
1. What innohep® is and what it is used for
innohep® is a type of heparin - a low molecular weight heparin - and belongs to a group of medicines called anticoagulants; these medicines affect how the blood clots.
innohep® prevents clotting, allowing normal blood flow through the arteries and veins.
innohep® is used in adults to treat:
• Harmful blood clots that have formed in a deep vein (deep vein thrombosis, DVT).
This usually occurs in a leg.
• Clots that may travel in your bloodstream and cause a blockage (a thromboembolism).
• A clot that has travelled to the lung and caused a blockage (pulmonary embolism, PE). This can cause breathing difficulties and chest pain.
• Blood clots that have developed because of the presence of a solid tumour (a particular type of cancer).
2. What you need to know before you use innohep®
Do not use innohep®
• If you are allergic (hypersensitive) to tinzaparin or any of the other ingredients of this medicine; you can find a list of ingredients in section 6 of this leaflet.
• If you have ever had a reaction to heparin that caused a severe drop in the number of your clotting cells (platelets) - this reaction is called heparin-induced thrombocytopenia (HIT).
• If you have ever had a major bleed (for instance: in the brain, spine, eye or stomach, into a muscle or the womb, or any conditions which make you bleed severely, such as haemophilia).
• If you have a condition called septic endocarditis (an inflammation of the lining of the heart and heart valves).
Important: You must not have an epidural/spinal anaesthetic within 24 hours after your last injection of innohep®. You must wait at least 4 to 6 hours after having a spinal anaesthetic, or after the catheter has been removed, before you start using innohep® again.
You may have a blood test before you start using this medicine and at intervals while you are using it; this is to check the level of the clotting cells (platelets) and potassium in your blood.
Warnings and precautions
Do not inject innohep® into a muscle. See section 3, “How to use innohep®”.
This medicine may make you bleed more easily, so when you are being given other injections or having any procedures carried out, tell the doctor, nurse or dentist that you are using innohep®.
If you have a brain tumour, you will be monitored closely because medicines such as innohep® that prevent blood from forming clots, may cause bleeding into the skull.
Talk to your doctor, pharmacist or nurse before using innohep®
• If you are pregnant or think you may be pregnant. See the section “Pregnancy and breast-feeding”.
• If you have a condition which makes you more likely to bleed.
• If you are being treated with other injections into your muscles.
• If you are allergic (hypersensitive) to heparin.
• If you are allergic to other low molecular weight heparins, such as enoxaparin or dalteparin.
• If you have a medical condition such as diabetes mellitus or metabolic acidosis which may cause high levels of potassium in your blood (hyperkalaemia).
• If you have an artificial heart valve.
• If you have kidney problems.
• If you have asthma, as this medicine contains sodium metabisulphite (see “Important information about some of the ingredients of innohep®” below).
innohep® should not be interchanged with other low molecular weight heparin products. This is because they are not exactly the same and you could experience problems with your blood clotting.
Elderly people
Because kidney problems are more likely if you are elderly, you may have a blood test to check how well your kidneys are working and to monitor the activity of innohep®.
Children and adolescents
innohep® is not intended for use in children and adolescents under the age of 18 years.
Other medicines and innohep®
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes any medicines bought without a prescription.
You must tell your doctor or pharmacist if you are taking any of the following medicines as you may be likely to bleed more easily:
• Non-steroidal anti-inflammatory drugs (such as ibuprofen or diclofenac): for arthritis or aches or pains.
• Aspirin: either for reducing pain and inflammation, or the lower dose for thinning of the blood.
• Platelet aggregation inhibitors (such as clopidogrel): for stopping harmful blood clots forming.
• Thrombolytic agents (such as streptokinase): for dissolving blood clots.
• Vitamin K antagonists (such as warfarin): for stopping harmful blood clots.
• Activated protein C: for getting rid of blood clots.
• Anticoagulation, taken by mouth (such as rivaroxaban, dabigatran or apixaban) for stopping harmful blood clots.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor, pharmacist or midwife for advice before using this medicine.
You must not have an epidural anaesthetic to help with your labour or surgery within 24 hours of your last injection of innohep®.
You must wait at least 4 to 6 hours after having a spinal anaesthetic, or after the catheter has been removed, before you start using innohep® again.
Driving and using machines
This medicine should not have any effect on your ability to drive or use machines.
However, you should check with your doctor if you feel any side effect that may stop you from driving or using machines.
Important information about some of the ingredients of innohep®
innohep® syringe contains:
• Sodium metabisulphite. This is a preservative and can cause an allergic reaction which may result in breathing difficulties. See section 4.
• Sodium. This medicine is nearly “sodium free”. Your medicine contains less than 23 milligrams (mg) of sodium per dose.
Please ask your doctor or pharmacist if you are worried about any of the ingredients in this medicine.
3. How to use innohep®
Your doctor may decide that you or a carer may inject this medicine. You will be shown how to do the injection and should only do the injection when you have been instructed how to do so.
Always use this medicine exactly as your doctor, pharmacist or nurse has told you.
Check with one of them if you are not sure that you understand how to do the injection or if you are unsure about anything else to do with the medicine.
How much innohep® to use Adults, including the elderly:
The dose depends on your weight and this will be worked out by the doctor who prescribes it for you. You will be told how much you need to inject. It is possible you may not need to inject the entire contents of the syringe.
You will have one dose of innohep® each day for at least 6 days.
The treatment may be continued for up to 6 months if you have a solid tumour (a particular type of cancer).
Use in children and adolescents
There is limited experience of use in children and adolescents. innohep® is not intended for use in children and adolescents under the age of l8 years.
How to inject yourself with innohep®
You should inject yourself exactly as you have been shown and only on the parts of your body that you have been told it is safe to inject into. The type of injection you will be giving is known as a subcutaneous injection. The injection goes into a pinched up fatty layer on your abdomen or on the outer part of your thighs. Keep away from your belly button. Do NOT inject into a muscle.
Ideally you should inject at the same time every day; this helps to maintain a steady level of the medicine in your body.
When giving yourself an injection, make sure you:
1. Thoroughly wash and dry your hands.
2. Sit, stand or lie in a position so that you can see the skin where you are going to inject yourself. This can comfortably be done standing up or, if you prefer, in a lounge chair, recliner or bed propped up with pillows.
3. Decide where to inject yourself. This is usually on the right or left side of the abdomen, remembering not to inject within 5 cm (2 inches) of your belly button. You may also inject into the sides of your thigh. Do not inject near any scars or bruises. Each time you inject yourself, choose the opposite side from the site of your previous injection.
So if you injected your right thigh last time, you would inject your left thigh next time. If you are injecting in your abdomen, you would do the left side one day and right the next. You should also avoid injecting into the exact site of a previous injection.
4. Clean the chosen area of the skin, as you have been told to do by your doctor or nurse, and allow to dry before you inject.
Carefully take the syringe out of its plastic container by bending the cap all the way back and sliding the syringe out. Bend the orange safety device down away from the cap on the needle. Remove the protective needle cap without bending the needle. To keep the needle clean, make sure it does not touch anything. The syringe is now ready for use. There is no need to remove the air bubble if you need the total quantity in the syringe for your dose.
5. IMPORTANT. If the dose you have been told to have is less than the amount in the syringe, you will need to get rid of the extra before your injection. To do this, hold the syringe vertically with the needle pointing upwards and gently remove the excess by pressing the plunger into the syringe.
6. Hold the syringe in your writing-hand like you would hold a pen. With your other hand, make a fold of your skin by gently pinching the area where you are going to inject yourself with your thumb and forefinger.
7. With the syringe at a right angle to your body (pointing straight, not at an angle), insert the needle fully into the skin fold.
8. Continue to hold the skin fold, press down on the plunger slowly over 10-15 seconds. This delivers the medicine into the fatty tissue.
9. Pull the needle completely out of the skin and then let go of the skin fold. Do not rub or massage the place where you injected yourself - this can lead to bruising.
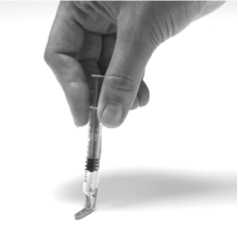

10. Using a hard surface, bend the orange safety device so it is now pointing in the same direction as the needle (back to its original position). Then with the safety device flat against a hard surface such as a table, gently push downwards until the needle clicks into the device. Then continue to push downwards against the hard surface, so that the needle and device are at a 45 degree angle to the syringe.
11. The used syringe, even when the orange safety device is in place, should be handled with care and should be disposed of in a “sharps” container (i.e. a special bin for needles) immediately. If a “sharps” container is not readily available then please put the used syringe back into the plastic container and close the lid by pressing down on the lid until it clicks into the slot provided. Dispose of the syringe carefully, as instructed by your healthcare professional.
Never put syringes or needles in the household rubbish.
For the attention of the healthcare professional:
Please dispose of the used syringe in accordance with your institution/employer's standard procedures for disposal of used syringes.
If you use more innohep® than you should
If you think you may have injected yourself with too much, tell your doctor or nurse straight away because you may start to haemorrhage (bleed severely) and need to be given another injection of a medicine called protamine sulphate to stop you bleeding.
If you have missed a dose of innohep®
If you forget to have your injection, it is important that you talk to your doctor or nurse as soon as you remember and get advice on what to do.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects most often reported are blood problems and skin reactions, especially where your injection has been given.
Important side effects to look out for:
You must get urgent medical help if you have any of the following symptoms. You may be having a serious allergic reaction. These are rare (may affect up to 1 in 1000 people)
• You have difficulty breathing.
• Your face or throat swell.
• Your skin develops a severe rash.
• You experience blistering of the skin, mouth, eyes or genitals or your skin peels.
• Blood spots near the injection site which could develop into a purple blister surrounded by red inflamed skin.
You must get urgent medical help if you have any of the following symptoms after having an epidural or spinal anaesthetic. You may be developing paralysis:
• Tingling, weakness or numbness in your legs or lower body.
• Back pain.
• Problems in going to the toilet.
You should tell your doctor straight away if you spot any of the following signs which mean you may be starting to bleed severely:
• Red or brown urine.
• Black tarry stools.
• Unusual bruising.
• Bleeding from your nose, or mouth or any operation wound that will not stop.
Common side effects (may affect up to 1 in 10 people)
• Bleeding (haemorrhage).
• Anaemia. Reduction in red blood cells which can make the skin pale and cause weakness and breathlessness.
• A pooling of blood in tissues which may result in the skin appearing dark in colour, similar to a large bruise.
• Pain, itching, bruising or bleeding, redness, swelling, nodules or hard lumps under your skin where the injection was given.
Uncommon side effects (may affect up to 1 in 100 people)
• Changes in your blood test results. There may be a change in the clotting cells (platelets) in your blood. These tests will return to normal when innohep® is stopped.
• An allergic reaction. You may be sensitive to one of the ingredients in this medicine.
• Bruising, red or purple spots under your skin.
• Some blood tests may also show a change in the way your liver is working. These tests will return to normal when innohep® is stopped.
• An itchy red rash with heat and swelling on your skin (dermatitis).
• Rash.
• Itchy skin.
Rare side effects (may affect up to 1 in 1000 people)
• Your blood may form more harmful clots. A drop in the number of clotting cells (platelets) in your blood may give you these symptoms. Your doctor can explain this more.
• Changes in your blood test results. The amount of potassium may be increased.
This is more likely to happen if you have severe kidney problems or diabetes. Your doctor can explain this more.
• Hives.
• Your bones may weaken and break more easily. This is known as osteoporosis and has been seen in patients using heparin for a long time.
• Prolonged, painful erections in men.
Paediatric population
Limited information derived from one study and postmarketing data indicates that the pattern of adverse reactions in children and adolescents is comparable to that in adults.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store innohep®
• Keep out of the sight and reach of children.
• Do not use after the expiry date on the syringe. The expiry date is the last day of that month.
• Do not store above 25°C.
• Medicines should not be thrown away in waste water or in household waste. Please ask your pharmacist how to throw away any medicine you do not need anymore. If you do this you will help protect the environment.
6. Contents of the pack and other information What innohep® contains
The active ingredient is tinzaparin sodium.
A pre-filled variable dose graduated syringe containing tinzaparin sodium 20,000 anti-Factor Xa IU in 1 ml.
• Each pre-filled variable dose graduated syringe of 0.5 ml contains tinzaparin sodium 10,000 anti-Factor Xa IU.
• Each pre-filled variable dose graduated syringe of 0.7 ml contains tinzaparin sodium 14,000 anti-Factor Xa IU.
Each pre-filled variable dose graduated syringe of 0.9 ml contains tinzaparin sodium 18,000 anti-Factor Xa IU.
• The other ingredients are sodium metabisulphite E223, sodium hydroxide and water for injection.
You can find important information about some of the ingredients near the end of section 2, just before section 3.
What innohep® looks like and contents of the pack
innohep® is a clear to straw coloured solution.
innohep® comes in a pre-filled variable dose graduated syringe with green plunger in plastic container and orange needle safety device with red lid containing 0.5 ml solution for injection.
innohep® comes in a pre-filled variable dose graduated glass syringe with green plunger and orange needle safety device in plastic container with yellow lid containing 0.7 ml solution for injection.
innohep® comes in a pre-filled variable dose graduated glass syringe with green plunger and orange needle safety device in plastic container with dark blue lid containing 0.9 ml solution for injection.
Available in packs of 2, 6 and 10 pre-filled syringes in a carton.
Not all pack sizes may be marketed.
Manufacturer and Product Licence Holder
Manufactured by Laboratoires LEO S.A., 39 Route de Chartres, F-28500 Vernouillet, France. Procured from within the EU by Product Licence holder: Star Pharmaceuticals Ltd., 5 Sandridge Close, Harrow, Middlesex HA1 1XD. Repackaged by Servipharm Ltd.
| POM |P PL 20636/2506
Leaflet revision and issue date (Ref.) 16.07.15[4] innohep® is a registered trademark of LEO Pharma A/S.