Invita D3 25 000 Iu Oral Solution
PACKAGE LEAFLET: INFORMATION FOR THE USER

*
CONSILI6NT H6HLTH u*
invitaD3
25,
000 IU oral solution
Cholecalciferol
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist, or nurse.
• This medicine has been prescribed for you only. Do not pass it onto others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What invitaD3 is and what it is used for
2. What you need to know before you use invitaD3
3. How to use invitaD3
4. Possible side effects
5. How to store invitaD3
6. Contents of the pack and other information
invitaD3 is a vitamin product containing cholecalciferol (equivalent to vitamin D3). Vitamin D can be found in some foods and also produced by the body when skin is exposed to sunlight. Vitamin D helps the kidneys and intestine absorb calcium and it helps build bones.
invitaD3 25,000 IU is used:
• for the prevention of vitamin D deficiency when there is a significant risk of deficiency or an increased demand for vitamin D
• with other medicines to treat certain bone conditions, such as thinning of the bone (osteoporosis)
• to treat vitamin D deficiency that has been confirmed by laboratory tests.
Do not use invitaD3
• if you are allergic to vitamin D or any of the other ingredients of invitaD3 (listed in section 6)
• if you have hypercalcaemia (high levels of calcium in the blood)
• if you have hypercalciuria (high levels of calcium in the urine)
• if you have pseudohypoparathyroidism (disturbed parathyroid hormone metabolism)
• if you have renal calculi (kidney stones)
• if you have hypervitaminosis D (high levels of vitamin D in the blood).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using invitaD3 if you:
• are undergoing treatment with certain medicines used to treat heart disorders (e.g. cardiac glycosides, such as digoxin)
• have sarcoidosis (an immune system disorder which may cause increased levels of vitamin D in the body)
• are taking medicines containing vitamin D, or eating foods or milk enriched with vitamin D
• are likely to be exposed to a lot of sunshine whilst using invitaD3
• take additional supplements containing calcium. Your doctor will monitor your blood levels of calcium to make sure they are not too high whilst you are using invitaD3
• have kidney damage or disease. Your doctor may want to measure the levels of calcium in your blood or urine.
Other medicines and invitaD3
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This is especially important if you are taking:
• medicines that act on the heart or kidneys, such as cardiac glycosides (e.g. digoxin) or diuretics (e.g. bendroflumethazide). When used at the same time as vitamin D these medicines may cause a large increase in the level of calcium in the blood and urine
• medicines containing vitamin D or eating food rich in vitamin D, such as, some types of vitamin D-enriched milk
• actinomycin (a medicine used to treat some forms of cancer) and imidazole antifungals (e.g. clotrimazole and ketoconazole, medicines used to treat fungal disease). These medicines may interfere with the way your body process vitamin D
• the following medicines because they can interfere with the effect or the absorption of vitamin D:
- antiepileptic medicines (anticonvulsants), barbiturates
- glucocorticoids (steroid hormones such as hydrocortisone or prednisolone). These can decrease the effect of vitamin D
- medicines that lower the level of cholesterol in the blood (such as cholestyramine, or colestipol)
- certain medicines for weight loss that reduce the amount of fat your body absorbs (e.g. orlistat)
- certain laxatives (such as liquid paraffin). invitaD3 with food, drink and alcohol
You should take this medicine preferably together with a large meal to help your body absorb the vitamin D. You can also mix the solution with cold or lukewarm food, to help you take this medicine. For detailed information see section 3 "How to use invitaDS”.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
This high strength formulation is not recommended for use in pregnant and breastfeeding women. Driving and using machines
There is limited information on the possible effects of this medicine on your ability to drive. However, it is not expected that it would affect your ability to drive or to operate machinery.
Always take invitaD3 exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
You should take invitaD3 preferably together with a large meal.
This medicine has a delicate taste of olive oil. The contents of the single-dose oral solution is to be emptied directly into the mouth and swallowed orally as per the diagram below.
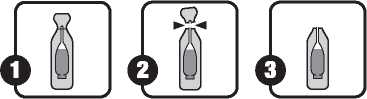
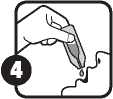
To help you take this medicine, the full contents of the single-dose oral solution may be emptied onto a spoon and taken orally. You may also mix the contents of the single-dose oral solution with a small amount of cold or lukewarm food immediately before use. Make sure the entire dose is taken.
Use in children and adolescents
The recommended dose for:
• Prevention of vitamin D deficiency in 0-1 years:
1 single-dose oral solution of invitaD3 25,000 IU every 8 weeks
• Prevention of vitamin D deficiency in 1-18 years:
1 single-dose oral solution of invitaD3 25,000 IU every 6 weeks
• Treatment of vitamin D deficiency in 0-18 years:
1 single-dose oral solution of invitaD3 25,000 IU once every 2 weeks for 6 weeks (followed by maintenance therapy of 400-1000 lU/day).
In children, invitaD3 can be mixed with a small amount of children's foods, yogurt, milk, cheese or other dairy products. Do not mix this medicine into a bottle of milk or container of soft food, in case your child does not consume the whole portion, and does not receive the full dose. You should make sure that the entire dose is taken. For children who are no longer breast-feeding you should give the prescribed dose with a substantial meal.
Do not store any product or food mixture that contains invitaD3 for use at a later time or a next meal.
Use in pregnancy and breast-feeding
This high strength formulation is not recommended.
Use in adults
The recommended dose for:
• Prevention of vitamin D deficiency:
1 single-dose oral solution of invitaD3 25,000 IU once monthly. Higher doses may be required based on the advice of your doctor.
• Addition to specific therapy for osteoporosis:
1 single-dose oral solution of invitaD3 25,000 IU once monthly.
• Treatment of vitamin D deficiency:
2 single-dose oral solutions of invitaD3 25,000 IU once weekly for 6-8 weeks, followed by maintenance therapy (1400-2000 IU once daily may be required), based on the advice of your doctor.
If you take more invitaD3 than you should
If you or your child takes more medicine than prescribed, stop using this medicine and contact your doctor. If it is not possible to talk to a doctor, go to the nearest hospital emergency department and take the medicine package with you.
The most common symptoms of overdose are: nausea, vomiting, excessive thirst, the production of large amounts of urine over 24 hours, constipation and dehydration, high levels of calcium in the blood (hypercalcaemia and hypercalciuria) shown by lab test.
If you forget to take invitaD3
If you forget to take a dose of invitaD3, take the forgotten dose as soon as possible. Then take the next dose at the correct time. However, if it is almost time to take the next dose, do not take the dose you have missed; just take the next dose as normal.
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Possible side effects may include:
Uncommon (affects less than 1 in 100 people)
• Too much calcium in your blood (hypercalcaemia)
• Too much calcium in your urine (hypercalciuria)
Rare (affects less than 1 in 1000 people)
• Skin rash
• Itching
• Hives
Reporting of side effects
If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effect directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE INVITAD3
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after "Exp”. The expiry date refers to the last day of that month.
Do not store above 30°C. Store invitaD3 in the original carton to protect the contents from light. Do not freeze or refrigerate.
Do not throw away any medicine via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What invitaD3 contains
• The active substance is cholecalciferol (vitamin D3).
• The other ingredients are tocopherol acetate, polyglyceryl oleate (E475), olive oil, refined, sweet orange peel oil.
What invitaD3 looks like and contents of the pack
invitaD3 is a clear, slightly yellow, oily liquid. It is supplied in transparent PVC/PVDC/PE singledose oral solution.
invitaD3 is available in packs of 3 PVC/PVDC/PE single-dose oral solutions.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
Consilient Health Limited,
5th Floor, Beaux Lane House, Mercer Street Lower, Dublin 2, Ireland.
Manufacturer
SMB Technology S.A.
39, rue du Parc Industriel, 6900 Marche en Famenne, Belgium.
This leaflet was last revised in January 2016.
P0410