Ipratropium Bromide 21 Micrograms Per Metered Dose Nasal Spray Solution
Out of date information, search anotherPackage Leaflet: Information for the user
Package Leaflet: Information for the user
Rinatec® 21 micrograms per metered dose Nasal Spray solution
(ipratropium bromide)
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets troublesome or serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Rinatec is and what it is used for
2. Before you use Rinatec
3. How to use Rinatec
4. Possible side effects
5. How to store Rinatec
6. Further information
1. What Rinatec is and what it is used for
The name of your medicine is Rinatec 21 micrograms per metered dose Nasal Spray solution (called Rinatec in this leaflet). It is a solution that you spray into your nose.
Rinatec contains a medicine called ipratropium bromide. This belongs to a group of medicines called ‘anticholinergics'. It is used to help stop a runny nose caused by Rhinitis. Rhinitis is an inflammation of the nose which could be because of an allergy (such as hay fever) or other reasons.
2. Before you use Rinatec Do not use Rinatec if:
• You are allergic (hypersensitive) to ipratropium bromide or any of the other ingredients in Rinatec (listed in Section 6 below)
• You are allergic (hypersensitive) to medicines that are similar to Rinatec, such as atropine
Do not use this medicine if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using Rinatec.
Take special care with Rinatec
Check with your doctor or pharmacist before using your medicine if:
• You have cystic fibrosis
• You have glaucoma or any other eye problems
• You are a man who has prostate problems
• You have problems passing water (urine)
• You are pregnant, likely to get pregnant or you are breastfeeding
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using Rinatec.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines. This includes medicines obtained without a prescription and herbal medicines. This is because Rinatec can affect the way some other medicines work.
Also some other medicines can affect the way Rinatec works.
Pregnancy and breast-feeding
Talk to your doctor or pharmacist before using this medicine if you are pregnant, likely to get pregnant or are breast-feeding.
Driving and using machines
You may feel dizzy, or have difficulty in focusing, or blurred vision while taking Rinatec. If this happens do not drive or use any tools or machines.
Important information about some of the ingredients of Rinatec
Rinatec contains benzalkonium chloride which is an irritant to the skin and may cause skin reactions.
3. How to use Rinatec
Always use Rinatec as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How many sprays Adults
• Two sprays in each nostril
• Two to three times each day
Children under 12 years of age
Rinatec is not recommended for children under 12 years
Using this medicine
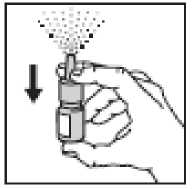
1. Remove the cap
2. • The nasal spray pump must be
primed before Rinatec is used for the first time
• To prime the pump, hold the bottle with your thumb at the base and your first and middle fingers on the white shoulder area. Point the bottle upright and away from your eyes.
Press your thumb firmly and quickly against the bottle seven times. The pump is now primed and ready to use.
If it has been more than 24 hours since you used your spray, you will need to prime the pump again before using it. Reprime the pump as before, but this time you will only need to pump the bottle twice
If you have not used your spray for more than seven days you will need to reprime the pump using the normal seven sprays
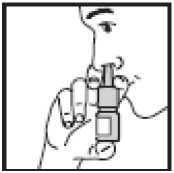
3. Blow your nose to clear your nostrils, if necessary.
4. • Close one nostril by gently placing
your finger against the side of your nose
• Tilt your head slightly forward and, keeping the bottle upright, insert the bottle tip into the other nostril. Point the tip toward the back and outer side of your nose
• Press firmly and quickly upwards with the thumb at the base while holding the white shoulder area between your first and middle fingers
• Following each spray, sniff deeply and breathe out through your mouth. After you have finished spraying, remove the bottle and tilt your head backwards for a few seconds to let the spray spread over the back of the nose
5. Repeat step 4 in the other nostril
6. Replace the cap
7. • If the bottle tip becomes clogged, remove cap. Hold the bottle
tip under warm running water for about a minute
• Dry the bottle tip, reprime the pump and replace the cap
If any of the spray accidentally gets into your eyes, wash it out with cold tap water for several minutes. You may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If affected, do not drive or use any tools or machines. If you get problems with your eyes at any other time, talk to your doctor for advice
If you use more RINATEC than you should
Do not use more sprays than the doctor has told you. If you use more sprays than you should, talk to a doctor or go to a hospital straight away. Take the medicine pack with you, even if there is no medicine left.
If you forget to use Rinatec
• If you forget a dose, use the spray as soon as you remember
• However, if it is nearly time for the next dose, skip the missed dose
• Do not use a double dose to make up for a forgotten dose
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Rinatec can cause side effects, although not everybody gets them. The following side effects may happen with this medicine:
Stop taking and see a doctor straight away, if you notice any of the following serious side effects - you may need urgent medical treatment.
• Allergic reactions - the signs may include itching or nettle rash (affects less than 1 in 1000 people). In severe cases the signs include swelling of your mouth and face, skin rash, sudden difficulties in breathing and reduction of your blood pressure.
Tightening of your throat (affects less than 1 in 100 people)
• Increased heart rate, irregular heart rhythm such as atrial fibrillation or quickening of the heart rate (affects less than 1 in 100 people)
• Palpitations (fast or uneven heart beats) (affects less than 1 in 1000 people)
Stop using this medicine and see your doctor straight away if you have any of these side effects.
Other side effects include:
Common (affects less than 1 in 10 people)
• Headache or stuffy nose
• Nasal drying, nasal irritation such as itching or a burning sensation or nosebleeds
• Throat irritation
Uncommon (affects less than 1 in 100 people)
• Skin rash
• Dizziness
• Unexpected tightness of the chest, swelling of the throat, dry throat
• Blurred vision, difficulty focusing, dilated pupils, glaucoma, painful, stinging, red or swelling of the eyes, see colours or lights
• Mouth or lip sores
• Problems passing water (urine), especially if you already have problems passing urine
• Feeling sick (nausea), stomach upset or discomfort
• Dry mouth
Rare (affects less than 1 in 1000 people)
• Itching or nettle rash (urticaria)
If any of the spray accidentally gets into your eyes, wash it out with cold tap water for several minutes. You may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If you get problems with your eyes at any other time, talk to your doctor for advice.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Rinatec
Keep out of the sight and reach of children.
Use this spray within six weeks of first priming the nasal spray pump. Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiry date refers to the last day of the month. Do not store above 25°C. Do not freeze. Do not expose to excessive heat.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information What Rinatec contains
Each metered dose contains 21 micrograms of the active ingredient ipratropium bromide (as monohydrate).
The other ingredients are: sodium chloride, benzalkonium chloride, disodium edetate, purified water, sodium hydroxide and hydrochloric acid.
What Rinatec looks like and contents of the pack
Rinatec is a clear colourless aqueous solution in an amber coloured bottle fitted with a white manually activated nasal pump/closure.
Rinatec is available as a 15 ml spray, which gives 180 individual sprays.
PL 20774/1379 - Rinatec 21 micrograms per metered dose Nasal Spray solution
' POM
Manufactured by: Istituto de Angeli S.r.l. Localita Prulli Reggello (FI), Italy. Procured from within the EU. Product Licence Holder: Quadrant Pharmaceuticals Ltd, Lynstock House, Lynstock Way, Lostock, Bolton, BL6 4SA. Repackaged by: Maxearn Ltd, Bolton, BL6 4SA.
Leaflet revised 14th April 2015
Rinatec is a registered trademark of Boehringer Ingelheim KG.
PP2/1379/V1
ready to use.
Ipratropium Bromide 21 micrograms per metered dose Nasal Spray solution
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets troublesome or serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Ipratropium Bromide is and what it is used for
2. Before you use Ipratropium Bromide
3. How to use Ipratropium Bromide
4. Possible side effects
5. How to store Ipratropium Bromide
6. Further information
1. What Ipratropium Bromide is and what it is used for
The name of your medicine is Ipratropium Bromide 21 micrograms per metered dose Nasal Spray solution (called Ipratropium Bromide in this leaflet). It is a solution that you spray into your nose.
Ipratropium Bromide contains a medicine called ipratropium bromide.
This belongs to a group of medicines called ‘anticholinergics'. It is used to help stop a runny nose caused by Rhinitis. Rhinitis is an inflammation of the nose which could be because of an allergy (such as hay fever) or other reasons.
2. Before you use Ipratropium Bromide Do not use Ipratropium Bromide if:
• You are allergic (hypersensitive) to ipratropium bromide or any of the other ingredients in Ipratropium Bromide (listed in Section 6 below)
• You are allergic (hypersensitive) to medicines that are similar to Ipratropium Bromide, such as atropine
Do not use this medicine if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using Ipratropium Bromide.
Take special care with Ipratropium Bromide
Check with your doctor or pharmacist before using your medicine if:
• You have cystic fibrosis
• You have glaucoma or any other eye problems
• You are a man who has prostate problems
• You have problems passing water (urine)
• You are pregnant, likely to get pregnant or you are breastfeeding
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using Ipratropium Bromide.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines. This includes medicines obtained without a prescription and herbal medicines. This is because Ipratropium Bromide can affect the way some other medicines work.
Also some other medicines can affect the way Ipratropium Bromide works.
Pregnancy and breast-feeding
Talk to your doctor or pharmacist before using this medicine if you are pregnant, likely to get pregnant or are breast-feeding.
Driving and using machines
You may feel dizzy, or have difficulty in focusing, or blurred vision while taking Ipratropium Bromide. If this happens do not drive or use any tools or machines.
Important information about some of the ingredients of Ipratropium Bromide
Ipratropium Bromide contains benzalkonium chloride which is an irritant to the skin and may cause skin reactions.
3. How to use Ipratropium Bromide
Always use Ipratropium Bromide as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How many sprays Adults
• Two sprays in each nostril
• Two to three times each day
Children under 12 years of age
Ipratropium Bromide is not recommended for children under 12 years
Using this medicine
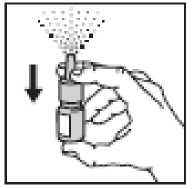
1. Remove the cap
2. • The nasal spray pump must be
primed before Ipratropium Bromide is used for the first time • To prime the pump, hold the bottle with your thumb at the base and your first and middle fingers on the white shoulder area. Point the bottle upright and away from your eyes.
Press your thumb firmly and quickly against the bottle seven times. The pump is now primed and
• If it has been more than 24 hours since you used your spray, you will need to prime the pump again before using it. Reprime the pump as before, but this time you will only need to pump the bottle twice
• If you have not used your spray for more than seven days you will need to reprime the pump using the normal seven sprays
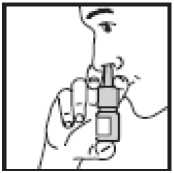
3. Blow your nose to clear your nostrils, if necessary.
4. • Close one nostril by gently placing
your finger against the side of your nose
• Tilt your head slightly forward and, keeping the bottle upright, insert the bottle tip into the other nostril. Point the tip toward the back and outer side of your nose
• Press firmly and quickly upwards with the thumb at the base
• while holding the white shoulder area between your first and middle fingers
• Following each spray, sniff deeply and breathe out through your mouth. After you have finished spraying, remove the bottle and tilt your head backwards for a few seconds to let the spray spread over the back of the nose
5. Repeat step 4 in the other nostril
6. Replace the cap
7. • If the bottle tip becomes clogged, remove cap. Hold the bottle
tip under warm running water for about a minute
• Dry the bottle tip, reprime the pump and replace the cap
If any of the spray accidentally gets into your eyes, wash it out with cold tap water for several minutes. You may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If affected, do not drive or use any tools or machines. If you get problems with your eyes at any other time, talk to your doctor for advice
If you use more IPRATROPIUM BROMIDE than you should
Do not use more sprays than the doctor has told you. If you use more sprays than you should, talk to a doctor or go to a hospital straight away. Take the medicine pack with you, even if there is no medicine left.
If you forget to use Ipratropium Bromide
• If you forget a dose, use the spray as soon as you remember
• However, if it is nearly time for the next dose, skip the missed dose
• Do not use a double dose to make up for a forgotten dose
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Ipratropium Bromide can cause side effects, although not everybody gets them. The following side effects may happen with this medicine:
Stop taking and see a doctor straight away, if you notice any of the following serious side effects - you may need urgent medical treatment.
• Allergic reactions - the signs may include itching or nettle rash (affects less than 1 in 1000 people). In severe cases the signs include swelling of your mouth and face, skin rash, sudden difficulties in breathing and reduction of your blood pressure.
Tightening of your throat (affects less than 1 in 100 people)
• Increased heart rate, irregular heart rhythm such as atrial fibrillation or quickening of the heart rate (affects less than 1 in 100 people)
• Palpitations (fast or uneven heart beats) (affects less than 1 in 1000 people)
Stop using this medicine and see your doctor straight away if you have any of these side effects.
Other side effects include:
Common (affects less than 1 in 10 people)
• Headache or stuffy nose
• Nasal drying, nasal irritation such as itching or a burning sensation or nosebleeds
• Throat irritation
Uncommon (affects less than 1 in 100 people)
• Skin rash
• Dizziness
• Unexpected tightness of the chest, swelling of the throat, dry throat
• Blurred vision, difficulty focusing, dilated pupils, glaucoma, painful, stinging, red or swelling of the eyes, see colours or lights
• Mouth or lip sores
• Problems passing water (urine), especially if you already have problems passing urine
• Feeling sick (nausea), stomach upset or discomfort
• Dry mouth
Rare (affects less than 1 in 1000 people)
• Itching or nettle rash (urticaria)
If any of the spray accidentally gets into your eyes, wash it out with cold tap water for several minutes. You may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If you get problems with your eyes at any other time, talk to your doctor for advice.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ipratropium Bromide
Keep out of the sight and reach of children.
Use this spray within six weeks of first priming the nasal spray pump. Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiry date refers to the last day of the month. Do not store above 25°C. Do not freeze. Do not expose to excessive heat.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information
What Ipratropium Bromide contains
Each metered dose contains 21 micrograms of the active ingredient ipratropium bromide (as monohydrate).
The other ingredients are: sodium chloride, benzalkonium chloride, disodium edetate, purified water, sodium hydroxide and hydrochloric acid.
What Ipratropium Bromide looks like and contents of the pack
Ipratropium Bromide is a clear colourless aqueous solution in an amber coloured bottle fitted with a white manually activated nasal pump/closure.
Ipratropium Bromide is available as a 15 ml spray, which gives 180 individual sprays.
PL 20774/1379 - Ipratropium Bromide 21 micrograms per metered dose Nasal Spray solution
POM
Manufactured by: Istituto de Angeli S.r.l. Localita Prulli Reggello (FI), Italy. Procured from within the EU. Product Licence Holder: Quadrant Pharmaceuticals Ltd, Lynstock House, Lynstock Way, Lostock, Bolton, BL6 4SA. Repackaged by: Maxearn Ltd, Bolton, BL6 4SA.
Leaflet revised 14th April 2015
PP1/1379/I/V2