Ipravent Cfc-Free Inhaler 20 Micrograms Per Actuation Pressurised Inhalation Solution
Out of date information, search anotherPackage leaflet: Information for the patient Ipravent CFC-Free Inhaler 20 micrograms per actuation pressurised inhalation, solution
(ipratropium bromide)
The name of this medicine is 'Ipravent CFC-Free Inhaler 20 micrograms per actuation pressurised inhalation, solution' which will be referred to as 'Ipravent Inhaler' throughout this leaflet.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or asthma nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms and signs of illness are the same as yours.
- if you get any side effects, talk to your doctor, pharmacist or asthma nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Ipravent Inhaler is and what it is used for
2. What you need to know before you use Ipravent Inhaler
3. How to use Ipravent Inhaler
4. Possible side effects
5. How to store Ipravent Inhaler
6. Contents of the pack and other information
1. What Ipravent Inhaler is and what it is used for
Ipravent Inhaler contains a medicine called ipratropium bromide. This belongs to a group of medicines called bronchodilators. It is used to make breathing easier for people with asthma or 'chronic obstructive pulmonary disease' (COPD), often referred to as chronic bronchitis.
If you have asthma or COPD, you may have difficulty in breathing, shortness of breath, wheezing or tightness in your chest.
Ipravent works by opening up airways.
2. What you need to know before you use Ipravent Inhaler
Do not use Ipravent Inhaler:
• If you are allergic (hypersensitive) to ipratropium bromide or any of the other ingredients of this medicine (listed in section 6).
• If you are allergic to similar medicines which contain atropine or medicines like atropine.
• If you are pregnant, think you are pregnant, are likely to get pregnant or are breastfeeding.
Do not use if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using Ipravent Inhaler.
Warnings and precautions
Talk to your doctor or pharmacist or asthma nurse before using Ipravent Inhaler if:
• You have glaucoma, or have been told that you may develop it.
• You have problems passing water (urine).
• You are a man who has prostate problems.
• You have cystic fibrosis.
• If you have liver or kidney problems.
If you are not sure if any of these apply to you, talk to your doctor or pharmacist or asthma nurse before using Ipravent Inhaler.
Ipratropium bromide is slow acting drug and therefore you will not get immediate relief after starting this medicine.
Talk to your doctor, pharmacist, or asthma nurse:
• If you are required to take a fast-acting reliever inhaler such as salbutamol more frequently than normal or if you are not getting relief from your reliever inhaler or if your symptoms are getting worse, this may be due to worsening of asthma and you should see your doctor straightaway.
• If your asthma seems to be getting worse you may have a bacterial infection and you must see your doctor. Your doctor may prescribe you antibiotics, may increase the dose of your inhaled corticosteroids and /or may start short course of oral
corticosteroids.
• If you experience allergic reactions like skin rash and itching (urticaria) or a severe allergic reactions (anaphylaxis) with swelling of your mouth, tongue and lips and swelling of your face, sudden difficulties in breathing (bronchospasm) and/or a tightness in your throat you must see your doctor immediately.
• If you experience sudden difficulty in breathing, increased wheezing or shortness of breath after taking a dose of Ipravent inhaler you must stop using your Ipravent Inhaler and not use it again and you must use your fast-acting reliever inhaler straightaway. You must contact your doctor immediately.
• If any of the spray from the inhaler accidently gets into your eyes you must contact your doctor or asthma nurse as soon as possible.
Children and adolescents:
Ipravent inhaler should not be used in children 12 years of age and younger or adolescents 13 to 17 years of age.
Other medicines and Ipravent Inhaler
Tell your doctor or pharmacist if you are taking, have recently taken or might use any other medicines, including any inhalers or medicines obtained without a prescription. This includes herbal medicines. This is because Ipravent Inhaler can affect the way some other medicines work. Also some other medicines can affect the way Ipravent Inhaler works.
In particular, tell your doctor or pharmacist if you are taking/have taken any of the following medicines:
• Other inhalers to help you breathe more easily such as the reliever inhaler salbutamol.
• Medicines called 'Xanthines' to help your breathing such as theophylline and aminophylline.
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using Ipravent Inhaler.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.The effect of Ipravent Inhaler on fertility has not been studied and there are no fertility data available.
Driving and using machines
You may feel dizzy, or have difficulty focusing, dilated pupils, blurred vision, visual halos and/or coloured images, red eyes, eye pain or discomfort when using Ipravent Inhaler. If any of these symptoms occur do not drive or use any tools or machines. If your eyes are affected in any way at all do not drive or operate machinery and contact your doctor straightaway.
Operations
If you attend a hospital appointment or are admitted to hospital be sure to take your inhaler(s) and any other medicines (in their packaging if possible) with you. Some gases used in operations (anaesthetics gases) may affect how your inhaler works. If you are about to have surgery, make sure you mention that you are using Ipravent Inhaler to the doctor, dentist or anaesthetist.
Ipravent Inhaler contains ethanol (alcohol)
This medicinal product contains a small amount of ethanol (8.4 mg in one single dose).
3. How to use Ipravent Inhaler
Always use Ipravent Inhaler exactly as your doctor has told you. Check with your doctor if you are not sure.
Use in children:
This pressurised metered dose inhaler is not to be used in children 12 years of age and younger or in adolescents 13 to 17 years of age.
Use in adults (including the elderly) with either asthma or COPD :
• 1 or 2 puffs to be inhaled three or four times daily.
• However, in early treatment some patients may need up to 4 puffs at a time to obtain maximum benefit.
Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or asthma nurse has told you. Check with your doctor, pharmacist or asthma nurse if your not sure.
Testing your Inhaler
When you use the inhaler for the first time you should test it to check that it works properly. Also do this if you have not used the inhaler for 3 or more days.
1. Remove the mouthpiece cover by gently pressing the sides with your thumb and index finger.
2. Point the mouthpiece away from you and press the canister twice (once at a time) to release two puffs into the air.
Using your Inhaler
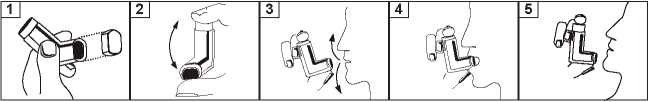
1. Stand or sit upright while using the Ipravent Inhaler
2. Remove the mouthpiece cover.
3. Check inside and outside the mouthpiece of the inhaler to make sure that it is clean and free from dust and dirt and any loose objects before inhaling.
4. Hold the inhaler upright between the thumb(s) on the base of the mouthpiece and a forefinger (s)/index finger(s) on the top of the inhaler (the arrow on the base of the container should be pointing upwards) and breathe out slowly and deeply.
5. Immediately after breathing out place the mouthpiece in your mouth between your teeth and close your lips around it but do not bite it
6. Breathe in slowly and deeply, through the mouth and immediately after starting to breathe in press down firmly on the top of the inhaler to release one actuation (puff) and continue to breathe in steadily and deeply.
7. Hold your breath, remove the inhaler from the mouth and take away the finger from top of the inhaler. Continue to hold your breath for as long as is comfortable, for 10 seconds if possible.
8. Breathe out slowly. Do not breathe out into the inhaler.
9. If a second inhalation is required you should wait at least one minute and then repeat steps 4 to 8.
10. Replace the mouthpiece cover after use.
IMPORTANT
• Do not inhale too quickly, start breathing in as slowly and steadily as possible just before pressing the inhaler
• Do not rush through the entire procedure
• Elderly patients and patients with weak hands may use the inhaler with both hands
The mouthpiece has been designed specially for use with this product only. Do not use any other mouthpiece with the product and do not use the mouthpiece provided here with any other product.
Practice in front of a mirror the first few times. If you see “mist” coming from the top of your inhaler or the sides of your mouth you should start again.
A spacing device may be useful for patients with weak hands, particularly the elderly and adults who find it difficult to synchronise breathing in and actuation of the inhaler.
XXXXX
XXXXX
However this Ipravent Inhaler is NOT to be used with ANY spacing device. If a spacing device is required you will have to have your inhaler treatment changed to an alternative inhaler that can be used with a spacing device.
If the Inhaler has been exposed to low temperatures, the patient should take the metal canister out of the plastic case and warm it in their hands for a minimum of two minutes.
When using your Ipravent Inhaler take care not to let any of the spray enter your eyes. If any of the spray from the inhaler accidently gets into your eyes you may get painful eyes, stinging or red eyes, dilated pupils, blurred vision, see colors or lights or halos around lights. If you get any of the above side effects, rinse the eye with cold tap water and immediately talk to your doctor, pharmacist or asthma nurse for further advice.
Cleaning your Inhaler
To prevent your inhaler from getting blocked up it should be cleaned at least once a week.
• Remove the canister and green mouthpiece cover.
• Wash and clean the white mouthpiece in warm soapy water.
• Rinse in warm water and allow to air dry without using any heating system.
• Make sure the small hole in the mouthpiece is washed through thoroughly.
• Once the mouthpiece is dry, replace the canister and the mouthpiece cover. Do not put the metal canister into water.
Do not use more than your doctor has told you to use See your doctor straightaway if:
• You feel that your inhaler is not working as well as usual. or
• You need to use the inhaler more than your doctor has recommended.
Your doctor may need to check how well your medicine is working. Your doctor may need to change your medicine.
Make sure you do not run out of Ipravent
The inhaler has been designed to deliver 200 puffs of your medicine. However, it is not possible to tell when the inhaler is empty and when the 200 puffs have been used. There may still be a small amount of fluid left over in the container. Please make sure that your inhaler is replaced after you have taken 200 puffs (usually after 3-4 weeks of regular use) so that you can be certain that you are getting the right amount of your medicine in each puff.
If you use more Ipravent than you should
If you use more of this medicine than you should, talk to a doctor or go to a hospital straightaway. Take all your inhalers and any other medicines you are taking (in their packaging if possible) with you. If you take too much or too many puffs you may get a dry mouth, a rapid heart rate or blurred vision.
If you forget to use your Ipravent Inhaler
• If you forget a dose, take it as soon as you remember it.
• However, if it is time for the next dose, skip the missed dose.
• Do not take a double dose to make up for a forgotten dose
If you have any further questions, ask your doctor or pharmacist or asthma nurse.
Do not stop using your Ipravent Inhaler unless your doctor tells you to.
If you have any further questions on the use of this medicine ask your doctor or pharmacist or asthma nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor straightaway if you notice any of the following serious side effects - you may need urgent medical treatment:
• If after using Ipravent Inhaler you are wheezy or have other difficulties in breathing, do not use any more (unless you have been told to by your doctor). You may need to use a fast-acting reliever inhaler such as salbutamol to help your breathing. Your doctor may decide that you need different medicines to help your breathing.
• As with other inhaled medicines you may experience difficulty in breathing with an immediate worsening of your wheezing after taking a dose of this medicine. This is known as paradoxical bronchospasm and will respond to a fast-acting 'reliever' inhaler, your 'blue' inhaler and should be treated straightaway. You should stop using Ipravent Inhaler immediately and contact the doctor. The doctor will want to see you and examine your chest and will probably prescribe a different inhaler to treat your asthma or COPD or chronic bronchitis.
Paradoxical bronchospasm is uncommon and affects less than 1 in 100 people.
• Allergic reactions - the signs may include skin rash and itching . In severe reactions the signs include swelling of your mouth (tongue and lips) and face, sudden difficulties in breathing and a fall in your blood pressure. Tightening of your throat may occur.. If allergic reactions occur, Ipravent should be stopped immediately; do not use your Ipravent Inhaler again; talk to your doctor or pharmacist straightaway.
Allergic reactions are uncommon and affects less than 1 in 100 people, talk to your doctor or pharmacist immediately.
• Palpitations (fast or uneven heart beats ) or quickening of the heart rate (affects less than 1 in 100 people).
• Increased heart rate or irregular heart rhythm such as atrial fibrillation (affects less than 1 in 1000 people).
• If any of the spray from the inhaler accidently gets into your eyes you may get painful eyes, stinging or red eyes, dilated pupils, blurred vision, see colors or lights or halos around lights. If this happens, talk to your doctor immediately for advice. If you get problems with your eyes at any other time also talk to your doctor for advice. You may be developing increased pressure in your eyes (glaucoma), which will need treatment (straightaway). If your eyes are affected in any way do not drive or operate machinery
See your doctor straightaway if you have any of these side effects.
The side effects described below have been experienced by people taking Ipravent Inhaler and they are listed as either common, uncommon or rare. Common (affects less than 1 in 10 people)
• Headache, dizziness
• Dry mouth, feeling sick (nausea), stomach upset or discomfort.
• Cough and throat irritation when you have just used Ipravent Inhaler Uncommon (affects less than 1 in 100 people)
• Itching, skin rash.
• Unexpected tightness of the chest, swelling of the throat, dry throat.
• Blurred vision, dilated pupils,increased pressure in the eye (glaucoma), painful eyes, stinging redness or swelling of the eyes, seeing colors, lights or halos around lights.
• Diarrhoea, constipation or being sick (vomitting).
• Mouth or lip sores.
• Problems passing water (urine), especially if you already have problems passing urine.
Rare (affects less than 1 in 1000 people)
• Difficulty focusing
• Nettle rash (urticaria)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Ipravent Inhaler
Keep this medicine out of the sight and reach of children
Do not use Ipravent Inhaler after the expiry date which is stated on the carton and canister label after the letters EXP The expiry date refers to the last day of that month.
Ipravent Inhaler should not be stored above 25°C. Keep away from direct sunlight and heat. Protect from frost.
If the Inhaler has been exposed to low temperatures, the patient should take the metal canister out of the plastic case and warm it in their hands for a minimum of two minutes.
The canister contains a pressurised liquid. Do not expose to temperatures higher than 50°C. Do not try to open, puncture or burn the canister even when apparently empty.
Do not throw away any medicines or inhalers via waste water or household waste. Ask your pharmacist how to throw away medicines and inhalers that you no longer use. These measures will help protect the environment
6. Contents of the pack and other information
What Ipravent Inhaler contains
• The active substance is ipratropium bromide.
One metered dose (ex-valve) contains 20 micrograms ipratropium bromide (as the monohydrate).This is equivalent to a delivered dose (ex-actuator) of 18 micrograms ipratropium bromide (as the monohydrate). Each canister contains 200 actuations, each actuation containing 20 micrograms ipratropium bromide (as the monohydrate).
• The other ingredients are CFC-Free propellant, propellant HFA 134a (1,1,1,2- tetrafluoroethane), citric acid anhydrous, ethanol anhydrous and purified water. Ipravent Inhaler does not contain any chlorofluorocarbon (CFC) propellants.
What Ipravent Inhaler looks like and contents of pack.
Ipravent Inhaler is comprised of a pressurised aluminium canister which contains pressurised solution for inhalation sealed with a metering valve made up of a thermoplastic body and a metallic ferrule and spring and a plastic actuator made up of polypropylene having a white mouthpiece fitted with a green mouthpiece cover. Each carton contains one inhaler.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Cipla (EU) Limited. Hillbrow House, Hillbrow Road, Esher, Surrey, KT10 9NW, United Kingdom
Manufacturer:
S&D Pharma CZ, spol. s r.o, Theodor 28, 273 08 Pchery (Pharmos a.s. facility), Ceska Republika
Cipla (EU) Limited, 4th Floor, 1 Kingdom Street, London, W2 6BY, United Kingdom
This medicinal product is authorised in the Member States of the EEA under the following names:
IE Ipragard Inhaler CFC-free 20 micrograms per metered dose, pressurised inhalation, solution
UK Ipravent CFC-Free Inhaler 20 micrograms per actuation pressurised inhalation, solution
This leaflet was last revised in 01/2014.
XXXXX