Izinova Concentrate For Oral Solution
Out of date information, search another1033192 RV - Izinova UK:1033192 RV - Izinova UK 31/10/13 10:01 Pagel
Package leaflet: information for the user
° IZINOVA®
concentrate for oral solution
Sodium sulfate anhydrous, magnesium sulfate heptahydrate and potassium sulfate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
• You must talk to a doctor if you do not feel better or if you feel worse.
What is in this leaflet
1. What Izinova is and what it is used for
2. What you need to know before you take Izinova
3. How to take Izinova
4. Possible side effects
5. How to store Izinova
6. Contents of the pack and other information
1. What izinova is and what it is used for
Izinova contains three different active substances called: sodium sulfate, magnesium sulfate and potassium sulfate.
Izinova is used in adults to cleanse your bowel before a medical procedure or bowel surgery.
Izinova is not a treatment for constipation.
Izinova must be diluted before use with the amount of water stated in the method of administration (see section 3).
2. What you need to know before you take Izinova
Do not take Izinova if:
• You are allergic to sodium sulfate, magnesium sulfate or potassium sulfate or any of the other ingredients of this medicine (listed in Section 6).
• You have any of the following stomach or gut problems:
• a known or suspected obstruction in the gut
• a perforated gut wall
• a stomach emptying problem (such as gastroparesis)
• paralysis of the gut
• toxic colitis or toxic megacolon
• excessive vomiting.
• You are severely dehydrated.
• You have a severe heart problem (congestive heart failure).
• You have ascites (excessive build up of fluid in your abdomen).
• You have a severe kidney problem (severe renal insufficiency).
• You suffer from active inflammatory bowel disease (Crohn’s disease or ulcerative colitis).
If you are not sure, talk to your doctor before taking Izinova.
Warnings and precautions
Talk to your doctor before taking Izinova if:
• You are dehydrated (the signs may include dry mouth, feeling thirsty, headache, feeling dizzy, urinating less than usual, extreme fatigue, heart palpitations and confusion).
• You have ever had abnormal sodium or potassium levels in your blood.
• You have a heart problem.
Izinova may affect the rhythm of the heart due to changes in the levels of salts in the blood. Your doctor may need to monitor you during your treatment.
• You have a kidney problem.
• You have a liver problem.
• You have a“uric acid” problem (gout or any other).
• You have a problem swallowing.
• You have “reflux” where acid from your stomach goes into your food pipe.
• You are physically weak or in fragile health.
• You have reduced movement in part or all of your intestines (hypomotility).
• You have history of medical conditions or gastrointestinal surgery that predispose to hypomo-tility disorders.
If you are frail or elderly (65 years old or more), have a serious kidney, liver, or heart condition or if you are at risk of changes to levels of salts in your body (electrolyte imbalances) your doctor may decide to perform a specific monitoring before and after the procedure. You should also pay particular attention to the recommendations given in this section of the leaflet as well as in the section “Other medicines and Izinova” and “How to take Izinova”.
If you suffer from significant vomiting or if you feel symptoms of dehydration (e.g. dry mouth, feeling thirsty) after taking this medicine, please contact your doctor who should set up rehydration measures.
If any of the above apply to you (or you are not sure), talk to your doctor before taking Izinova.
You will have frequent, loose bowel movements after taking the medicine. This is normal and shows that the medicine is working. Make sure you stay near to a toilet until the effect of the medicine has worn off. You should follow exactly the instructions of use of Izinova and drink as much water or clear liquids as you need to stop you becoming dehydrated.
Children and adolescents Izinova is not for use in patients below 18 years old. Its safety and efficacy has not yet been established in this population.
Other medicines and Izinova
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines obtained without a prescription and herbal medicines.
If you are taking any other medicines take them one to three hours before taking Izinova or at least one hour after the end of your cleansing process. This is because the diarrhoea caused by Izinova can flush medicines from the body so they may not work as expected. Special care should be taken with:
• medicines which may change the levels of fluid or salts in the blood (such as diuretics, calcium channel blockers or lithium treatment) or medicines that affect the rhythm of the heart.
• medicines regularly taken by mouth: for example, oral contraceptives, medicines for epilepsy or diabetes or antibiotics, levothyroxine (a hormone to treat reduced function of the thyroid gland), or digoxine (used for heart problems) because Izinova may delay or completely prevent the absorption of these oral medicines, making them less effective or ineffective.
Pregnancy and breast-feeding
If you are pregnant or breastfeeding, think that you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
If you are breast feeding, you should not breast feed your child for 48 hours after taking the second dose of Izinova.
Driving and using machines Izinova is not likely to affect you being able to drive or use any tools or machines.
Izinova contains sodium and potassium
If you are on a controlled salt (sodium or potassium) diet, please note that each bottle of Izinova contains 5.683g (247.2 mmol) sodium and 1.405g (35.9 mmol) potassium.
3. How to take Izinova
Always take this medicine exactly as described in this leaflet or as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Taking this medicine
• Take this medicine by mouth.
• The day before your procedure you can have a light breakfast. After breakfast only have clear liquids for lunch, dinner and any other meals until the procedure is performed. Do not drink red and purple liquids, milk or alcoholic beverages.
• Izinova is provided as 2 bottles packed in a box with a cup for dilution and to give the medicine. You need all these for your treatment.
• Do not drink the content of both bottles at the same time.
• Do not drink the content of the bottles before you have diluted them.
• Do not forget to drink the extra amount of water or authorised clear liquid.
• Your doctor will give you a form to record the time you start the treatment and the amount of liquids you have drunk during the bowel cleansing preparation. You should follow the instructions exactly and drink as much water or clear liquids as you need to stop you becoming dehydrated.

This medicinal product is authorised in the Member States of the EEA under the following names: Izinova - France, United Kingdom, Italy
Eziclen - Belgium, Czech Republic, Estonia, Germany, Greece, Latvia, Lithuania, Luxembourg, Netherlands, Poland, Portugal, Romania, Spain
• “Clear liquids” are water, tea or coffee (no milk or non-dairy creamer), fizzy (carbonated) or still (non-carbonated) soft drinks, strained fruit juices without pulp (not coloured red or purple), clear soup or soup strained to remove any solids.
Do not drink any alcoholic beverages.
How and when to take this medicine
Izinova can either be taken as a “Two-day” or as a “One-day”
plan. Your doctor will decide which plan you should take and its timing. You need to complete your plan at least one hour before your procedure.
“Two-day” plan
The administration is divided between the evening before the procedure and the morning of the day of the procedure.
The day before the procedure:
• begin the first part of the plan (first bottle) in the early evening (i.e. not later than 6 pm).
The day of the procedure:
• begin the second part of the plan (second bottle) in the early morning, 10 to 12 hours after starting the first part of the plan (first bottle).
“One-day” plan
The administration is started and completed the evening before the procedure.
The day before the procedure:
• begin the first part of the plan (first bottle) in the early evening (i.e. not later than 6 pm).
• begin the second part of the plan (second bottle) about
2 hours after starting the first part of the plan (first bottle).
Whatever the plan selected by your doctor, the following steps should be followed for both the first and the second parts of the plan:
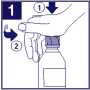
Open the childproof bottle by pressing down on the lid and twisting anticlockwise.
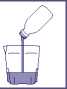
Pour the contents of one bottle of Izinova into the cup.

Add water to the medicine until the level reaches the line on the cup.

Take your time (over half an hour to an hour) to drink all the liquid in the cup.
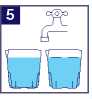
IMPORTANT: Drink two (2) more cups of water or clear liquid. Each time, fill the cup with water or clear liquid to the line.
6 2 Xtthr
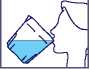
Take your time (over half an hour) to drink all the liquid of each cup.
Steps 1 to 6 should take around 2 hours and should be repeated for the second part of the plan.
What ever the plan, you should complete it at least one hour before your procedure.
If you take more Izinova than you should
• If you think you have taken too much Izinova, or did not dilute as instructed, or did not drink enough additional water, tell your doctor and drink sufficient water or clear liquids to stop you becoming dehydrated.
If you forget to take Izinova
• If you forget a dose, talk to your doctor as soon as possible as it may mean that the medicine will not work as expected.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine:
Stop taking Izinova and see a doctor straight away, if you notice any of the following:
• An allergic reaction - the signs may include skin rash or redness, itching, difficulty breathing or swelling of the throat.
Other side effects include:
Very common (may affect more than 1 in 10 people)
• General discomfort
• Feeling sick (nausea) or being sick (vomiting)
• Stomach bloating or pain.
Uncommon (may affect up to 1 in 100 people)
• Chills
• Dry mouth
• Headache
• Pain when urinating
• Discomfort in your anus or rectum
• Changes in the level of certain things in your blood. Examples include: increased “aspartate aminotransferase”, “creatine phosphokinase”, “lactate dehydrogenase”, “phosphorus”, “bilirubin” or “uric acid” and decreased “sodium”,
“potassium” or “calcium”.
Reporting of side effects If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Izinova
• Keep this medicine out of the sight and reach of children.
• Do not use this medicine after the expiry date which is stated on the carton and bottle after EXP. The expiry date refers to the last day of that month.
• This medicine does not require any special temperature storage conditions.
• Store in the original package, in order to protect from light.
• After first opening the bottle and/or diluting with water, the solution should be used immediately.
• Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Izinova contains
• The active substances are: sodium sulfate anhydrous, magnesium sulfate heptahydrate and potassium sulfate. Each bottle of about 176 ml concentrate contains 17.510 g sodium sulfate anhydrous, 3.276 g magnesium sulfate heptahydrate and 3.130 g potassium sulfate.
The total content of electrolyte ions is as follows:
|
Contents in g |
Contents in mmol | |||
|
1 bottle |
2 bottles |
1 bottle |
2 bottles | |
|
Sodium* |
5.683 |
11.366 |
247.2 |
494.4 |
|
Potassium |
1.405 |
2.81 |
35.9 |
71.8 |
|
Magnesium |
0.323 |
0.646 |
13.3 |
26.6 |
|
Sulfate |
14.844 |
29.688 |
154.5 |
309.0 |
* derived from sodium sulfate (active ingredient) and sodium benzoate (excipient).
1 The other ingredients are sodium benzoate (E211), citric acid anhydrous, malic acid, sucralose, purified water and fruit cocktail flavour (which contains natural and synthetic flavouring substances, propylene glycol E1520, ethyl alcohol, acetic acid and benzoic acid E210).
What Izinova looks like and contents of the pack
• This medicine is a concentrate for oral solution, the concentrate is clear or slightly hazy.
• It is provided as 2 bottles of about 176 ml each packed in a box with a cup, with a volume of about half a litre, for dilution and to give the medicine.
Marketing Authorisation Holder
Ipsen Limited 190 Bath Road Slough Berkshire SL1 3XE UK
Tel. 01753 627777
Manufacturer
Beaufour Ipsen Industrie
Rue Ethe Virton - 28100 Dreux
France
Tel. +33(0)2 37 65 46 00
Is this leaflet hard to see or read? Please phone 01753 627777 and ask for help.
This leaflet was last revised in 10/2013.
IP5EM

|
CLIENT |
BEAUFOUR IPSEN INDUSTRIE |
|
TRAVAIL |
Not. IZINOVA UK |
CAHIER DES CHARGES
|
QUANTITE | |
|
TOLERANCE DE LIVRAISON |
% |
|
QUALITE PAPIER |
VELIN |
|
SPECIFITE PAPIER | |
|
COULEUR PAPIER |
BLANC |
|
GRAMMAGE PAPIER |
50 g. |
|
FORMAT ROGNE |
180 x 315 |
|
LIVRE |
BOBINES |
|
LAIZE |
180 mm |
|
SPOTAGE |
Tous les 315 mm |
|
COULEUR(S) IMP |
BLEU - CYAN |
|
PANTONE |
2765 u |
|
RECTO ou R°-V° |
RECTO - VERSO |
|
ENROULEMENT |
N° 2 - R° Interieur |
|
SENS FIBRES | |
|
CARACTERES |
Corps 9 |
|
DIAM. MANDRIN | |
|
EMBALLAGE |
CARTONS -PAQUETS |
|
PORT |
DU
|
W. O. |
134419 |
|
CODE |
1033192 |
|
DATE |
31/10/2013 |
VOTRE COMMANDE N°
ssible
Il s'entend que nous ne pourrons en aucun cas etre tenus responsable des erreurs eventuelles que vous n'auriez pas decelees lors de ce bon a tirer.
SIGNATURE POUR BON A TIRER
apres corrections et compte tenu des observations ci-dessous
PHARPRINT SA - 28100 DREUX - TEL. 02 37 50 10 66 - FAX 02 37 50 18 08