Jaydess 13.5 Mg Intrauterine Delivery System
SUMMARY OF PRODUCT CHARACTERISTICS
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
1 NAME OF THE MEDICINAL PRODUCT
Jaydess 13.5 mg intrauterine delivery system.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
The intrauterine delivery system contains 13.5 mg levonorgestrel.
For the full list of excipients, see section 6.1.
For details of release rates, see section 5.2.
3 PHARMACEUTICAL FORM
Intrauterine delivery system (IUS).
The product consists of a whitish or pale yellow drug core covered with a semiopaque membrane, which is mounted on the vertical stem of a T-body. In addition, the vertical stem contains a silver ring located close to the horizontal arms. The T-body has a loop at one end of the vertical stem and two horizontal arms at the other end. Removal threads are attached to the loop. The vertical stem of the IUS is loaded in the insertion tube at the tip of the inserter. The IUS and inserter are essentially free of visible impurities.
Dimensions of Jaydess: 28 x 30 x 1.55 mm
4 CLINICAL PARTICULARS
4.1
Therapeutic indications
Contraception for up to 3 years.
4.2 Posology and method of administration
Posology
Jaydess is inserted into the uterine cavity and is effective for up to three years.
Insertion and removal/ replacement
It is recommended that Jaydess should only be inserted by physicians/ healthcare providers who are experienced in IUS insertions and/ or have undergone training on the Jaydess insertion procedure.
Jaydess is to be inserted into the uterine cavity within seven days of the onset of menstruation. Jaydess can be replaced by a new system at any time in the cycle. Jaydess can also be inserted immediately after first trimester abortion.
Postpartum insertions should be postponed until the uterus is fully involuted, however not earlier than six weeks after delivery. If involution is substantially delayed, consider waiting until 12 weeks postpartum.
In case of a difficult insertion and/ or exceptional pain or bleeding during or after insertion, appropriate steps should be taken immediately to exclude perforation, such as physical examination and ultrasound. Physical examination may not be sufficient to exclude partial perforation.
Jaydess can be distinguished from other IUSs by the visibility of the silver ring on ultrasound. The T-frame of Jaydess contains barium sulphate which makes it visible in X-ray examination.
Jaydess is removed by gently pulling on the threads with a forceps. If the threads are not visible and the system is found to be in the uterine cavity on ultrasound exam, it may be removed using a narrow forceps. This may require dilatation of the cervical canal or surgical intervention.
The system should be removed no later than by the end of the third year. If the woman wishes to continue using the same method, a new system can be inserted immediately following removal of the original system.
If pregnancy is not desired, the removal should be carried out within 7 days of the onset of menstruation, provided the woman is still experiencing regular menses. If the system is removed at some other time during the cycle and the woman has had intercourse within a week, she is at risk of pregnancy unless a new system is inserted immediately following removal.
After removal of Jaydess, the system should be examined to ensure that it is intact.
Elderly patients
Jaydess has not been studied in women over the age of 65 years. There is no indication for the use of Jaydess in postmenopausal women.
Patients with hepatic impairment
Jaydess has not been studied in women with hepatic impairment. Jaydess is contraindicated in women with acute liver disease or liver tumour (see section 4.3).
Patients with renal impairment
Jaydess has not been studied in women with renal impairment.
Paediatric population
Use of this product before menarche is not indicated. For data on safety and efficacy in adolescents, see section 5.1.
Method of administration
To be inserted by a healthcare provider using aseptic technique.
Jaydess is supplied within an inserter in a sterile package, which should not be opened until needed for insertion. Do not resterilise. As supplied, Jaydess is for single use only. Do not use if the blister is damaged or open. Do not insert after the expiry date which is stated on the carton and the blister after EXP.
Any unused product or waste material should be disposed of in accordance with local requirements.
Preparation for insertion
- Examine the patient to establish the size and position of the uterus, in order to detect any signs of acute genital infections or other contraindications for the insertion of Jaydess. If there is any doubt regarding pregnancy, a pregnancy test should be performed.
- Insert a speculum, visualize the cervix, and then thoroughly cleanse the cervix and vagina with a suitable antiseptic solution.
- Employ an assistant as necessary.
- Grasp the anterior lip of the cervix with a tenaculum or other forceps to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to grasp the posterior lip of the cervix. Gentle traction on the forceps can be applied to straighten the cervical canal. The forceps should remain in position and gentle counter traction on the cervix should be maintained throughout the insertion procedure.
- Advance a uterine sound through the cervical canal to the fundus to measure the depth and confirm the direction of the uterine cavity and to exclude any evidence of intrauterine abnormalities (e.g., septum, submucous fibroids) or a previously inserted intrauterine contraceptive which has not been removed. If difficulty is encountered, consider dilatation of the canal. If cervical dilatation is required, consider using analgesics and/or a paracervical block.
Insertion
1. First, open the sterile package completely (Figure 1). Then use aseptic technique and sterile gloves.
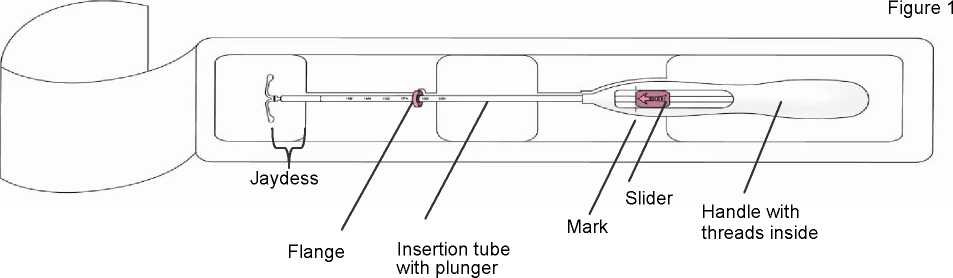
Figure 2
and scale
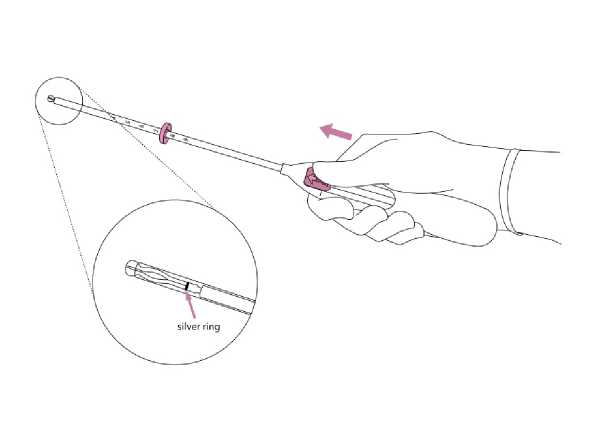
2. Push the slider forward in the direction of the arrow to the furthest position to load Jaydess into the insertion tube (Figure 2).
IMPORTANT! Do not pull the slider downwards as this may prematurely release Jaydess. Once released, Jaydess cannot be re-loaded.
3. Holding the slider in the furthest position, set the upper edge of the flange to correspond to the sound measurement of the uterine depth (Figure 3).
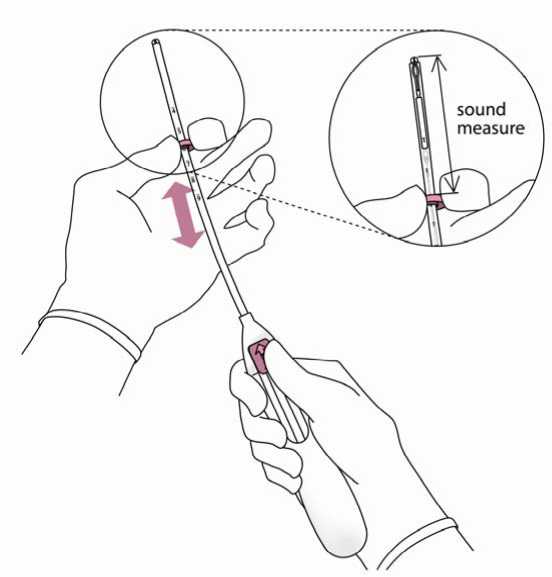
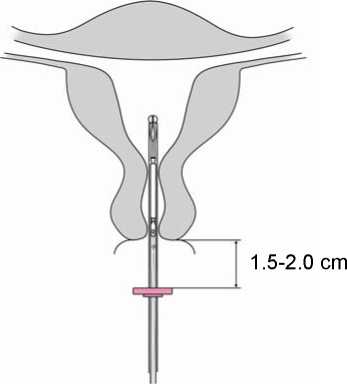
Figure 4
4. While holding the slider in the furthest position, advance the inserter through the cervix until the flange is approximately 1.5-2.0 cm from the uterine cervix (Figure
4).
IMPORTANT! Do not force the inserter. Dilate the cervical canal, if necessary.
5. While holding the inserter steady, pull the slider to the mark to open the horizontal arms of Jaydess (Figure 5). Wait 5 -10 seconds for the horizontal arms to open completely.
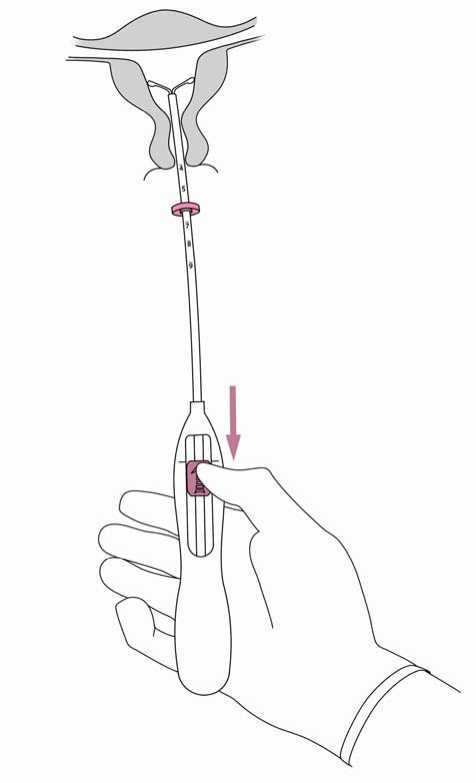
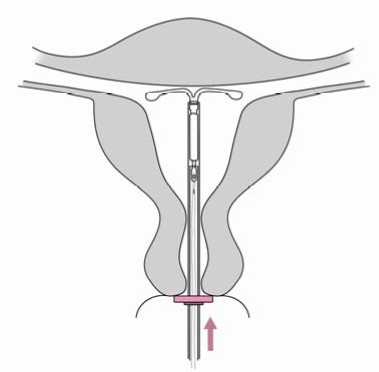
Figure 6
6. Advance the inserter gently towards the fundus of the uterus until the flange touches the cervix. Jaydess is now in the fundal position (Figure 6).
7. Holding the inserter in place, release Jaydess by pulling the slider all the way down (Figure 7). While holding the slider all the way down, gently remove the inserter by pulling it out.
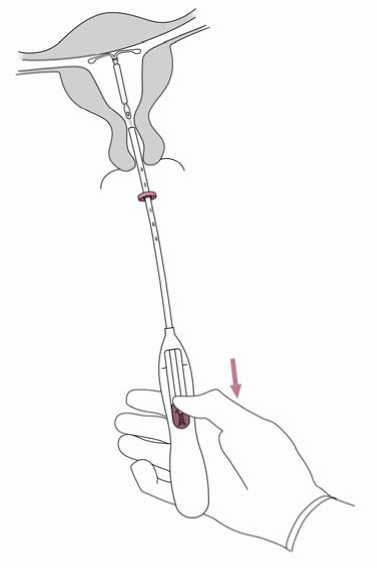
Cut the threads to leave about 2-3 cm visible outside of the cervix.
IMPORTANT! Should you suspect that the system is not in the correct position, check placement (e.g. with ultrasound). Remove the system if it is not positioned properly within the uterine cavity. A removed system must not be re-inserted.
Removal/ replacement
For removal/ replacement, please see section 4.2 Insertion and removal/ replacement.
Jaydess is removed by pulling on the threads with a forceps (Figure 8).
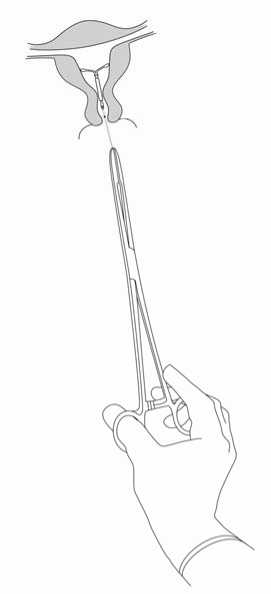
You may insert a new Jaydess immediately following removal.
After removal of Jaydess, the system should be examined to ensure that it is intact.
4.3 Contraindications
- Pregnancy (see section 4.6);
- Acute or recurrent pelvic inflammatory disease or conditions associated with increased risk for pelvic infections;
- Acute cervicitis or vaginitis;
- Postpartum endometritis or infected abortion during the past three months;
- Cervical intraepithelial neoplasia until resolved;
- Uterine or cervical malignancy;
- Progestogen-sensitive tumours, e.g. breast cancer;
- Abnormal vaginal bleeding of unknown etiology;
- Congenital or acquired uterine anomaly including fibroids which would interfere with insertion and / or retention of the intrauterine system (i.e. if they distort the uterine cavity);
- Acute liver disease or liver tumour;
- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Jaydess should be used with caution after specialist consultation, or removal of the system should be considered if any of the following conditions exist or arise for the first time:
- migraine, focal migraine with asymmetrical visual loss or other symptoms indicating transient cerebral ischemia
- exceptionally severe headache
- jaundice
- marked increase of blood pressure
- severe arterial disease such as stroke or myocardial infarction
Low-dose levonorgestrel may affect glucose tolerance, and the blood glucose concentration should be monitored in diabetic users of Jaydess. However, there is generally no need to alter the therapeutic regimen in diabetics using LNG IUS.
Medical examination/ consultation
Before insertion, a woman must be informed of the benefits and risks of Jaydess, including the signs and symptoms of perforation and the risk of ectopic pregnancy, see below. A physical examination including pelvic examination, examination of the breasts, and a cervical smear should be performed. Pregnancy and sexually transmitted diseases should be excluded. Genital infections should be successfully treated prior to insertion. The position of the uterus and the size of the uterine cavity should be determined. Fundal positioning of Jaydess is important in order to maximize the efficacy and reduce the risk of expulsion. The instructions for the insertion should be followed carefully.
Emphasis should be given to training in the correct insertion technique.
Insertion and removal may be associated with some pain and bleeding. The procedure may precipitate a vasovagal reaction (e.g. syncope, or a seizure in an epileptic patient).
A woman should be re-examined 4 to 6 weeks after insertion to check the threads and ensure that the system is in the correct position. Follow-up visits are recommended once a year thereafter, or more frequently if clinically indicated.
Jaydess is not for use as a post-coital contraceptive.
The use of Jaydess for the treatment of heavy menstrual bleeding or protection from endometrial hyperplasia during estrogen replacement therapy has not been established. Therefore it is not recommended for use in these conditions.
Ectopic pregnancy
In clinical trials, the overall incidence of ectopic pregnancy with Jaydess was approximately 0.11 per 100 woman-years. Approximately half of the pregnancies that occur during Jaydess use are likely to be ectopic.
Women considering Jaydess should be counselled on the signs, symptoms and risks of ectopic pregnancy. For women who become pregnant while using Jaydess, the possibility of an ectopic pregnancy must be considered and evaluated.
Women with a previous history of ectopic pregnancy, tubal surgery or pelvic infection carry an increased risk of ectopic pregnancy. The possibility of ectopic pregnancy should be considered in the case of lower abdominal pain, especially in connection with missed periods or if an amenorrheic woman starts bleeding.
Because an ectopic pregnancy may impact future fertility the benefits and risks of using Jaydess should be carefully evaluated, in particular for nulliparous women.
Use in nulliparous women: Jaydess is not first choice for contraception in nulliparous women as clinical experience is limited.
Effects on the menstrual bleeding pattern
Effects on the menstrual bleeding pattern are expected in most users of Jaydess. Those alterations are a result of the direct action of levonorgestrel on the endometrium and may not correlate with the ovarian activity.
Irregular bleeding and spotting are common in the first months of use. Thereafter, the strong suppression of the endometrium results in the reduction of the duration and volume of menstrual bleeding. Scanty flow frequently develops into oligomenorrhea or amenorrhea.
In clinical trials, infrequent bleeding and/ or amenorrhea developed gradually in about 22.3 % and 11.6 % of the users, respectively. Pregnancy should be considered if menstruation does not occur within six weeks of the onset of previous menstruation. A repeated pregnancy test is not necessary in subjects who remain amenorrheic unless indicated by other signs of pregnancy.
If bleeding becomes heavier and/ or more irregular over time, appropriate diagnostic measures should be taken as irregular bleeding may be a symptom of endometrial polyps, hyperplasia or cancer and heavy bleeding may be a sign of unnoticed expulsion of the IUS.
Pelvic infection
While Jaydess and the inserter as such are sterile they may, due to bacterial contamination during insertion, become a vehicle for microbial transport in the upper genital tract. Pelvic infection has been reported during use of any IUS or IUD. In clinical trials, pelvic inflammatory disease (PID) was observed more frequently at the beginning of Jaydess use, which is consistent with published data for copper IUDs, where the highest rate of PID occurs during the first 3 weeks after insertion and decreases thereafter.
Before electing use of Jaydess, patients should be fully evaluated for risk factors associated with pelvic infection (e.g. multiple sexual partners, sexually transmitted infections, prior history of PID). Pelvic infections such as PID may have serious consequences and it may impair fertility and increase the risk of ectopic pregnancy.
As with other gynaecological or surgical procedures, severe infection or sepsis (including group A streptococcal sepsis) can occur following IUD insertion, although this is extremely rare.
If a woman experiences recurrent endometritis or pelvic inflammatory disease or if an acute infection is severe or does not respond to treatment, Jaydess must be removed.
Bacteriological examinations are indicated and monitoring is recommended, even with discrete symptoms indicative of infections.
Expulsion
In clinical trials with Jaydess, the incidence of expulsion was low and in the same range as that reported for other IUDs and IUSs. Symptoms of the partial or complete expulsion of Jaydess may include bleeding or pain. However, partial or complete expulsion can occur without the woman noticing it, leading to decrease or loss of contraceptive protection. As Jaydess typically decreases menstrual bleeding over time, an increase of menstrual bleeding may be indicative of an expulsion.
A partially expelled Jaydess should be removed. A new system can be inserted at that time provided pregnancy has been excluded.
A woman should be advised how to check the threads of Jaydess and to contact her healthcare provider if the threads cannot be felt.
Perforation
Perforation or penetration of the uterine corpus or cervix by an intrauterine contraceptive may occur, most often during insertion, although it may not be detected until sometime later, and may decrease the effectiveness of Jaydess. In case of a difficult insertion and/ or exceptional pain or bleeding during or after insertion, appropriate steps should be taken immediately to exclude perforation, such as physical examination and ultrasound. Such a system must be removed; surgery may be required.
In a large prospective comparative non-interventional cohort study in users of other IUDs (N=61,448 women), the incidence of perforation was 1.3 (95% CI: 1.1 - 1.6) per 1000 insertions in the entire study cohort; 1.4 (95% CI: 1.1 - 1.8) per 1000 insertions in the cohort of another LNG- IUS and 1.1 (95% CI: 0.7 - 1.6) per 1000 insertions in the copper IUD cohort.
The study showed that both breastfeeding at the time of insertion and insertion up to 36 weeks after giving birth were associated with an increased risk of perforation (see Table 1). These risk factors were independent of the type of IUD inserted.
Table 1: Incidence of perforation per 1000 insertions for the entire study cohort, stratified by breastfeeding and time since delivery at insertion (parous women)
|
Breastfeeding at time of insertion |
Not breastfeeding at time of insertion | |
|
Insertion < 36 weeks |
5.6 |
1.7 |
|
after delivery | ||
|
95% CI: 3.9-7.9, |
95% CI: 0.8-3.1, | |
|
n=6047 insertions) |
n=5927 insertions) | |
|
Insertion > 36 weeks |
1.6 |
0.7 |
|
after delivery | ||
|
(95% CI: 0.0-9.1, |
(95% CI: 0.5-1.1, | |
|
n=608 insertions) |
n=41,910 insertions) |
The risk of perforations may be increased in women with fixed retroverted uterus.
Re-examination after insertion should follow the guidance given under the heading "Medical examination/consultation" which may be adapted as clinically indicated in women with risk factors for perforation.
Lost threads
If the removal threads are not visible at the cervix on follow-up examinations, unnoticed expulsion and pregnancy must be excluded. The threads may have been drawn up into the uterus or cervical canal and may reappear during the next menstrual period. If pregnancy has been excluded, the threads may usually be located by gently probing the cervical canal with a suitable instrument. If they cannot be found, the possibility of expulsion or perforation should be considered. Ultrasound exam may be used to ascertain the position of the system. If ultrasound is not available or is not successful, X-ray may be used to locate Jaydess.
Ovarian cysts / enlarged ovarian follicles
Since the contraceptive effect of Jaydess is mainly due to its local effects within the uterus, there is generally no change in ovulatory function, including regular follicular development, oocyte release and follicular atresia in women of fertile age. Sometimes atresia of the follicle is delayed and folliculogenesis may continue. These enlarged follicles cannot be distinguished clinically from ovarian cysts and have been reported in clinical trials as adverse drug events in approximately 13.2 % of women using Jaydess including ovarian cyst, hemorrhagic ovarian cyst and ruptured ovarian cyst. Most of these cysts are asymptomatic, although some may be accompanied by pelvic pain or dyspareunia.
In most cases, the enlarged follicles resolve spontaneously over two to three months observation. Should an enlarged follicle fail to resolve spontaneously, continued ultrasound monitoring and other diagnostic/ therapeutic measures may be appropriate. Rarely, surgical intervention may be required.
4.5 Interaction with other medicinal products and other forms of interaction
Interactions can occur with drugs that induce hepatic microsomal enzymes, specifically cytochrome P450 enzymes, and may therefore increase the metabolism of levonorgestrel, resulting in increased clearance of sex hormones (e.g. phenytoin, barbiturates, primidone, carbamazepine, rifampicin, rifabutin, nevirapine, efavirenz, bosentan, and possibly also oxcarbazepine, topiramate, felbamate, griseofulvin and products containing the herbal remedy St. John’s Wort).
On the other hand, substances known to inhibit drug-metabolizing enzymes (e.g. itraconazole, ketoconazole) may increase serum concentrations of levonorgestrel.
The influence of these drugs on the efficacy of Jaydess is not known, but it is not believed to be of major importance due to the local mechanism of action.
Magnetic resonance imaging (MRI)
Non-clinical testing has demonstrated that a patient can be scanned safely after placement of Jaydess under the following conditions: Static magnetic field of 3-Tesla or less, maximum spatial gradient magnetic field of 720-Gauss/cm or less. Under these conditions, with 15-min of scanning, the maximum temperature rise produced at the site of Jaydess was 1.8°C. A small amount of imaging artifact may occur if the area of interest is in the exact same area or relatively close to the position of Jaydess.
4.6 Fertility, pregnancy and lactation
Fertility
The use of a levonorgestrel-releasing intrauterine system does not alter the course of future fertility. Upon removal of the intrauterine system, women return to their normal fertility (see section 5.1).
Pregnancy
The insertion of Jaydess in pregnant women is contraindicated (see section 4.3).
If a woman becomes pregnant while using Jaydess ectopic pregnancy should be excluded and timely removal of the system is recommended since any intrauterine contraceptive left in situ may increase the risk of abortion and preterm labor. Removal of Jaydess or probing of the uterus may also result in spontaneous abortion. If the woman wishes to continue the pregnancy and the system cannot be withdrawn, she should be informed about the risks and the possible consequences of premature birth to the infant. The course of such a pregnancy should be closely monitored. The woman should be instructed to report all symptoms that suggest complications of the pregnancy, like cramping abdominal pain with fever.
Because of the intrauterine administration and the local exposure to LNG, the possible occurrence of virilizing effects in a female fetus should be taken into consideration. Clinical experience of the outcomes of pregnancies under Jaydess treatment is limited due to the high contraceptive efficacy. Women should be informed that, to date, there is no evidence of birth defects caused by a levonorgestrel-releasing intrauterine system use in cases where pregnancy has continued to term with the LNG-IUS in place.
Breastfeeding
In general, there appears to be no deleterious effect on infant growth or development when using any progestogen-only method after six weeks postpartum. A levonorgestrel-releasing intrauterine system does not affect the quantity or quality of breast milk. Small amounts of progestogen (about 0.1 % of the levonorgestrel dose) pass into the breast milk in nursing mothers.
4.7 Effects on ability to drive and use machines
Jaydess has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Summary of the safety profile
The majority of women experience changes in menstrual bleeding pattern after insertion of Jaydess. Over time, the frequency of amenorrhoea and infrequent bleeding increases, and the frequency of prolonged, irregular and frequent bleeding decreases. The following bleeding patterns were observed in clinical trials:
Table 2: Bleeding patterns reported with Jaydess in clinical trials
|
Jaydess |
First 90 days |
Second 90 days |
End of year 1 |
End of year 3 |
|
Amenorrhea |
<1% |
3% |
6% |
12% |
|
Infrequent bleeding |
8% |
19% |
20% |
22% |
|
Frequent bleeding |
31% |
12% |
8% |
4% |
|
Irregular bleeding* |
39% |
25% |
18% |
15% |
|
Prolonged bleeding* |
55% |
14% |
6% |
2% |
*Subjects with irregular bleeding and prolonged bleeding may also be included in one of the other categories (excluding amenorrhea)
Tabulated summary of adverse events
The frequencies of Adverse Drug Reactions (ADRs) reported with Jaydess are
summarised in the table below. Within each frequency grouping, undesirable effects
are presented in order of decreasing seriousness. Frequencies are defined as:
very common (> 1/10),
common (> 1/100 to < 1/10),
uncommon (> 1/1,000 to < 1/100),
rare (> 1/10,000 to < 1/1,000),
very rare (< 1/10,000).
|
System Organ Class |
Very Common |
Common |
Uncommon |
Rare |
|
Psychiatric disorders |
Depressed mood/ Depression | |||
|
Nervous system disorders |
Headache |
Migraine | ||
|
Gastrointestinal disorders |
Abdominal/pelvic pain |
Nausea | ||
|
Skin and subcutaneous tissue disorders |
Acne/ Seborrhoea |
Alopecia |
Hirsutism | |
|
Reproductive system and breast disorders |
Bleeding changes including increased and decreased menstrual bleeding, spotting, infrequent bleeding and amenorrhoea Ovarian cyst* Vulvovaginitis |
Upper genital tract infection Dysmenorrhea Breast pain/discomfort Device expulsion (complete and partial) Genital discharge |
Uterine perforation** |
* In clinical trials ovarian cysts had to be reported as AEs if they were abnormal, non-functional cysts and/or had a diameter > 3 cm on ultrasound examination.
** This frequency is based on clinical trials that excluded breastfeeding women. In a large prospective comparative non-interventional cohort study with women using another LNG-IUS and copper IUDs, the frequency of perforation in women who were breastfeeding or had an insertion up to 36 weeks after delivery was “uncommon” (see section 4.4 under Perforation).
Description of selected adverse reactions
With the use of another LNG-IUS, cases of hypersensitivity including rash, urticaria and angioedema have been reported.
If a woman becomes pregnant while using Jaydess, the relative likelihood of this pregnancy being ectopic is increased (see section 4.4 under Ectopic Pregnancy).
The removal threads may be felt by the partner during intercourse.
The following ADRs have been reported in connection with the insertion or removal procedure of Jaydess: Procedural pain, procedural bleeding, insertion-related vasovagal reaction with dizziness or syncope. The procedure may precipitate a seizure in an epileptic patient.
For other IUDs, cases of sepsis (including group A streptococcal sepsis) have been reported following insertion (see section 4.4 under Pelvic Infection).
Paediatric population
The safety profile of Jaydess observed in a study of 304 adolescents was consistent with that in the adult population.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme, Website: www.mhra.gov.uk/yellowcard
4.9 Overdose
Not relevant.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Plastic IUD with progestogen, ATC code: G02BA03 Pharmacodynamic effects
Jaydess has mainly local progestogenic effects in the uterine cavity.
The high levonorgestrel concentration in the endometrium down-regulates endometrial estrogen and progesterone receptors. The endometrium becomes relatively insensitive to the circulating estradiol and a strong antiproliferative effect is seen. Morphological changes of the endometrium and a weak local foreign body reaction are observed during use. Thickening of the cervical mucus prevents passage of the sperm through the cervical canal. The local milieu of the uterus and of the fallopian tubes inhibits sperm mobility and function, preventing fertilisation. In clinical trials with Jaydess ovulation was observed in the majority of the subjects studied. Evidence of ovulation was seen in 34 out of 35 women in the first year, in 26 out of 27 women in the second year, and in all 27 women in the third year.
Clinical efficacy and safety
The contraceptive efficacy of Jaydess has been evaluated in a clinical study with 1432 women aged 18-35 including 38.8 % (556) nulliparous women of whom 83.6% (465) were nulligravid using Jaydess. The 1-year Pearl Index was 0.41 (95% confidence limits 0.13 - 0.96) and the 3-years Pearl Index was 0.33 (95% confidence limits 0.16 - 0.60). The failure rate was approximately 0.4% at 1 year and the cumulative failure rate was approximately 0.9% at 3 years. The failure rate also includes pregnancies occurring after undetected expulsions and perforations. Use of a levonorgestrel releasing intrauterine system does not alter the course of future fertility. Based on data with a higher dosed LNG-IUS about 80% of the women wishing to become pregnant conceived within 12 months after removal of the system.
The safety profile of Jaydess observed in a study of 304 adolescents was consistent with that in the adult population. Efficacy is expected to be the same for adolescents under the age of 18 as for users 18 years and older.
With Jaydess, the alterations in menstrual patterns are a result of the direct action of levonorgestrel on the endometrium and may not reflect the ovarian cycle. There is no clear difference in follicle development, ovulation or estradiol and progesterone production in women with different bleeding patterns. In the process of inhibition of the endometrial proliferation, there can be an initial increase of spotting during the first months of use. Thereafter, the strong suppression of the endometrium results in the reduction of the duration and volume of menstrual bleeding during use of Jaydess. Scanty flow frequently develops into oligomenorrhea or amenorrhea. Ovarian function remains normal and estradiol levels are maintained, even when users of Jaydess are amenorrhoeic.
5.2 Pharmacokinetic properties
Levonorgestrel is released locally into the uterine cavity. The in vivo release curve is characterized by an initial steep decline that slows down progressively resulting in little change after 1 year until the end of the intended 3-year period of use. Estimated in vivo delivery rates for different time points are provided in Table 3.
Table 3: Estimated in vivo release rates based on observed ex vivo residual content data
|
Time |
Estimated in vivo release rate [micrograms/24 hours] |
|
24 days after insertion |
14 |
|
60 days after insertion |
10 |
|
1 year after insertion |
6 |
|
3 years after insertion |
5 |
|
Average over 3 years |
6 |
Absorption
Following insertion, levonorgestrel is released from the IUS into the uterine cavity without delay based on serum concentration measurements. Maximum serum concentrations of levonorgestrel are reached within the first two weeks after insertion of Jaydess. Seven days after insertion, a mean levonorgestrel concentration of 162 pg/ml was determined. Thereafter serum concentrations of levonorgestrel decline over time to reach mean concentrations of 59 pg/ml after 3 years. With the use of a levonorgetrel-releasing intrauterine system, the high local drug exposure in the uterine cavity leads to a strong concentration gradient from the endometrium to the myometrium (gradient endometrium to myometrium >100-fold), and to low concentrations of levonorgestrel in serum (gradient endometrium to serum >1000-fold).
Distribution
Levonorgestrel is bound non-specifically to serum albumin and specifically to SHBG. Less than 2 % of the circulating levonorgestrel is present as free steroid. Levonorgestrel binds with high affinity to SHBG. Accordingly, changes in the concentration of SHBG in serum result in an increase (at higher SHBG concentrations) or in a decrease (at lower SHBG concentrations) of the total levonorgestrel concentration in serum. Within one month after insertion of Jaydess, the concentration of SHBG declines by about 30%. Thereafter, plateau-like SHBG concentrations are observed with a tendency to increase towards baseline values over time. The mean apparent volume of distribution of levonorgestrel is about 106 L.
Biotransformation
Levonorgestrel is extensively metabolized. The major metabolites in plasma are the unconjugated and conjugated forms of 3a, 5^-tetrahydrolevonorgestrel. Based on in vitro and in vivo studies, CYP3A4 is the main enzyme involved in the metabolism of levonorgestrel.
Elimination
The total clearance of levonorgestrel from plasma is approximately 1.0 ml/min/kg. Only trace amounts of levonorgestrel are excreted in unchanged form. The metabolites are excreted in feces and urine at an excretion ratio of about 1. The excretion half-life is about 1 day.
Linearity/ non-linearity
The pharmacokinetics of levonorgestrel are dependent on the concentration of SHBG which itself is influenced by estrogens and androgens. During the first month of use of Jaydess, a mean SHBG decrease of about 30 % is observed which leads to a decrease of levonorgestrel in serum indicating non-linear pharmacokinetics of levonorgestrel with regard to time. Based on the mainly local action of Jaydess, no impact on the efficacy of Jaydess is expected.
Paediatric population
In a one-year phase III study in post-menarcheal female adolescents (mean age 16.2, range 12 to 18 years) pharmacokinetic analysis of 283 subjects showed estimated LNG serum concentrations slightly higher (10%) in adolescents compared to adults. This correlates to the generally lower body weight in adolescents. The ranges estimated for adolescents lie, however, within the ranges estimated for adults, showing high similarity.
5.3 Preclinical safety data
Nonclinical data revealed no special hazard for humans based on studies of safety pharmacology, pharmacokinetics and toxicity, including genotoxicity and carcinogenic potential of levonorgestrel. Studies in monkeys with intrauterine delivery of levonorgestrel for 9 to 12 months confirmed local pharmacological
activity with good local tolerance and no signs of systemic toxicity. No embryotoxicity was seen in the rabbit following intrauterine administration of levonorgestrel. The safety evaluation of the elastomer components of the hormone reservoir, the polyethylene materials of the product, the silver profile and the combination of elastomer and levonorgestrel, based on both the assessment of genetic toxicology in standard in vitro and in vivo test systems and on biocompatibility tests in mice, rats, guinea pigs, rabbits and in vitro test systems have not revealed bioincompatibility.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Polydimethylsiloxane elastomer
Silica, colloidal anhydrous
Polyethylene
Barium sulfate
Iron oxide, black (E172)
Silver
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
3 years.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
The product is individually packed into a thermoformed blister package (PETG) with a peelable lid (PE).
Pack sizes: 1x1 and 5x1.
Not all pack sizes may be marketed.
Special precautions for disposal
6.6
The product is supplied in a sterile pack which should not be opened until required for insertion. Each system should be handled with aseptic precautions. If the seal of the sterile envelope is broken, the system inside should be disposed of in accordance with local guidelines for the handling of biohazardous waste. Likewise, a removed Jaydess and inserter should be disposed of in this manner. The outer carton package and the inner blister package can be handled as household waste.
To be inserted by a healthcare provider using aseptic technique (see section 4.2).
7 MARKETING AUTHORISATION HOLDER
Bayer plc,
Bayer House,
Strawberry Hill,
Newbury,
Berkshire,
RG14 1JA
8 MARKETING AUTHORISATION NUMBER(S)
PL 00010/0587
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
25/01/2013
10 DATE OF REVISION OF THE TEXT
14/06/2016