Jext 300 Micrograms Solution For Injection In Pre-Filled Pen
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Jext 300 micrograms solution for injection in pre-filled pen
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Jext 300 micrograms: One pre-filled pen delivers one dose of 0.30ml solution for injection containing 300 micrograms of adrenaline (as tartrate).
1 ml solution contains 1mg adrenaline (as tartrate).
Excipients with known effect: Sodium metabisulphite (E223) and sodium chloride. Jext contains less than 1 mmol sodium (23 mg) per dose.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Solution for injection in pre-filled pen.
Clear and colourless solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Jext is indicated in the emergency treatment of severe acute allergic reactions (anaphylaxis) to insect stings or bites, foods, drugs and other allergens as well as idiopathic or exercise induced anaphylaxis.
4.2 Posology and method of administration
Posology
Paediatric population
Patients between 15 kg and 30 kg in weight:
The usual dose is 150 micrograms.
A dosage below 150 micrograms cannot be administered in sufficient accuracy in children weighing less than 15 kg and use is therefore not recommended unless life-threatening situation and under medical advice. Children and adolescents over 30 kg in weight should be prescribed a Jext 300 micrograms.
Use in adults over 30 kg in weight:
The usual dose is 300 micrograms.
Larger adults may require more than one injection to reverse the effect of an allergic reaction.
Patients between 15 kg and 30 kg in weight should be prescribed a Jext 150 micrograms.
An initial dose should be administered as soon as symptoms of anaphylaxis are recognised.
The effective dose is typically in the range of 0.005-0.01 mg/kg but higher doses may be necessary in some cases.
In the absence of clinical improvement or if deterioration occurs, a second injection with an additional Jext may be administered 5-15 minutes after the first injection. It is recommended that patients are prescribed two Jext pens which they should carry at all times.
Method of administration For intramuscular use.
For single use.
Jext is for intramuscular administration into the anterolateral thigh.
It is designed to inject through clothing or directly through the skin.
Massage around the injection area is advised to accelerate absorption.
Please refer to section 6.6 for detailed instructions for use.
The patient/carer should be informed that following each use of Jext:
• They should call for immediate medical assistance, ask for an ambulance and state ‘anaphylaxis’ even if symptoms appear to be improving (see section 4.4).
• Conscious patients should preferably lie flat with feet elevated but sit up if they have breathing difficulties. Unconscious patients should be placed on their side in the recovery position.
• The patient should if possible remain with another person until medical assistance arrives.
4.3 Contraindications
There are no absolute contraindications to the use of Jext during an allergic emergency.
4.4 Special warnings and precautions for use
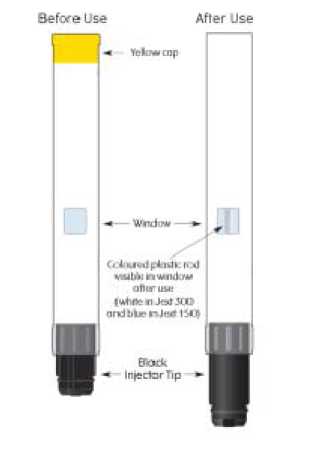
Do not remove yellow cap until ready for use.
Jext should be administered into the anterolateral thigh. The injection is delivered immediately after the black needle shield of the auto-injector is pressed firmly against the skin or other surface. Patients should be advised not to inject Jext into the gluteus maximus due to the risk of accidental injection into a vein.
The patient should be instructed to dial 999, ask for ambulance, state anaphylaxis to seek emergency medical assistance immediately after administering the first dose in order to have close monitoring of the anaphylactic episode and further treatment as required.
The patient/carer should be informed about the possibility of biphasic anaphylaxis which is characterised by initial resolution followed by recurrence of symptoms some hours later.
Patients with concomitant asthma may be at increased risk of a severe anaphylactic reaction.
Jext contains sodium metabisulphite which may rarely cause severe hypersensitivity reactions including anaphylactic symptoms and bronchospasm in susceptible people, especially those with a history of asthma. Patients with these conditions must be carefully instructed in regard to the circumstances under which Jext should be used.
Due to an increased risk of adverse reactions following administration of adrenaline special caution should be taken in patients with cardiovascular diseases including angina pectoris, obstructive cardiomyopathy, cardiac arrhythmia, cor pulmonale, atherosclerosis and hypertension.
Special caution should also be taken in patients with hyperthyroidism, phaeochromocytoma, narrow angle glaucoma, severe renal impairment, prostatic adenoma leading to residual urine, hypercalcaemia, hypokalaemia and diabetes. Caution should also be taken in elderly and pregnant patients.
In patients with thick sub-cutaneous fat layer, there is a risk for adrenaline not reaching the muscle tissue resulting in a suboptimal effect.
Peripheral ischaemia following accidental injection into hands or feet may cause loss of blood flow to adjacent areas due to vasoconstriction.
All patients who are prescribed Jext should be thoroughly instructed to understand the indications for the use and the correct method of administration (see section 6.6). It is strongly advised also to educate the patient’s immediate associates (e.g. parents, caregivers, teachers) for the correct usage of the Jext in case support is needed in the emergency situation.
There is often a prolonged period between supply of Jext and an allergic reaction requiring adrenaline. Patients should be advised to regularly check Jext and ensure it is replaced within the expiry period.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially sodium free.
Patients should be warned regarding related allergens and should be investigated whenever possible so that their specific allergens can be characterised.
4.5 Interaction with other medicinal products and other forms of interaction
Caution is indicated in patients receiving drugs that may sensitise the heart to arrhythmias, including digitalis and quinidine. The effects of adrenaline may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors (MAO-inhibitors) and catechol-O-methyl transferase inhibitors (COMT inhibitors).
Adrenaline inhibits the secretion of insulin, thus increasing the blood glucose level. It may be necessary for diabetic patients receiving adrenaline to increase their dosage of insulin or oral hypoglycaemic drugs.
The alpha- and beta-stimulating effect can be inhibited by concomitant use of alpha- and beta-blocking drugs as well as parasympathomimetic drugs.
4.6 Pregnancy and lactation
Clinical experience in the treatment of anaphylaxis during pregnancy is limited. Adrenaline should only be used during pregnancy if the potential benefit justifies the potential risk for the foetus.
Adrenaline is not orally bioavailable; any adrenaline excreted in breast milk would not be expected to have any effect on the nursing infant.
4.7 Effects on ability to drive and use machines
Jext has no or negligible influence on the ability to drive and use machines, however, patients are not recommended to drive or use machines following administration of adrenaline, since they will be affected by the anaphylactic reaction.
4.8 Undesirable effects
Side effects associated with adrenaline's alpha and beta receptor activity may include cardiovascular effects as well as undesirable effects on the central nervous system.
The following table is based upon post marketing experience with the use of adrenaline. The frequency cannot be estimated from the available data.
|
System Organ Class |
Adverse Drug Reaction |
|
Metabolism and nutrition disorders |
Hyperglycaemia, hypokalaemia, metabolic acidosis |
|
Psychiatric disorders |
Anxiety, hallucination |
|
Nervous system disorders |
Headache, dizziness, tremor, syncope |
|
Cardiac disorders |
Tachycardia, arrhythmia, palpitations, angina pectoris, stress cardiomyopathy |
|
Vascular disorders |
Hypertension, vasoconstriction, peripheral ischaemia |
|
Respiratory, thoracic and mediastinal disorders |
Bronchospasm |
|
Gastrointestinal disorders |
Nausea, vomiting |
|
General disorders and administration site conditions |
Hyperhidrosis, asthenia |
Peripheral ischaemia following accidental injection of adrenaline in the hands or feet has been reported.
Jext contains sodium metabisulphite which may rarely cause severe hypersensitivity reactions including anaphylactic symptoms and bronchospasm (see section 4.4. Special warning and precautions for use).
4.9 Overdose
Overdose or inadvertent intravascular injection of adrenaline may cause cerebral haemorrhage and ventricular arrhythmias resulting from a sharp rise in blood pressure. Myocardial ischaemias and necroses as well as renal impairment may occur. Fatalities may also result from pulmonary oedema because of peripheral vascular constriction together with cardiac stimulation.
Pulmonary oedema may be treated with alpha-blocking agents such as phentolamine. In case of arrhythmias these may be treated with beta-blocking agents.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Cardiac stimulants ex cl. cardiac glycosides, adrenergic and dopaminergic agents.
ATC code: C01CA24.
Adrenaline is a catecholamine which stimulates the sympathetic nervous system (both alpha and beta receptors) by which cardiac rate, cardiac output and coronary circulation is raised. Adrenaline through its action on beta receptors on bronchial smooth muscles causes bronchial smooth muscle relaxation which alleviates wheezing and dyspnoea.
5.2 Pharmacokinetic properties
Adrenaline is a naturally occurring substance produced by the adrenal medulla and secreted in response to exertion or stress. It is rapidly inactivated in the body mostly by the enzymes COMT and MAO. The liver is rich in these enzymes and is an important, although not essential, tissue in the degradation process. Much of the dose of adrenaline is accounted for by excretion of metabolites in the urine.
The plasma half-life of adrenaline is about 2.5 min. However local vasoconstriction may retard absorption, so that the effects can last longer than the half-life would predict. Massage around the injection area is advised to accelerate absorption.
5.3 Preclinical safety data
Adrenaline has been utilised in the treatment of allergic emergencies for many years. There is no preclinical data of relevance available.
6.1 List of excipients
Sodium Chloride
Sodium Metabisulphite (E223)
Hydrochloric Acid (for pH adjustment)
Water for Injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
18 months
6.4 Special precautions for storage
Store below 25°C. Do not freeze.
6.5 Nature and contents of container
Pre-filled pen (single dose pen) comprising an auto-injector with a cartridge. The cartridge is made of glass (type 1), sealed with a latex free grey rubber plunger and a latex free bromobutyl rubber seal within an anodised aluminium cap. The autoinjector is made of plastic.
Exposed needle length:
Jext 150 micrograms: 13 mm Jext 300 micrograms: 15 mm
Pack-size: Single pack with 1 pre-filled pen. Multipack with 2 pre-filled pens.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Jext is a single-use pre-filled pen designed for easy use.
The pre-filled pen is operated by simply pressing the black injector tip against the outer thigh. This will activate a plunger, which pushes a concealed needle through the membrane on the black injector tip into the muscle and injects a dose of adrenaline. This can be done through clothing.
Jext 300 micrograms contains 1.4 ml of adrenaline injection 1 mg/ml which is designed to deliver a single dose (0.30 ml) of 300 micrograms adrenaline when activated. After activation of the auto-injector 1.1 ml remains in the pre-filled pen. Discard any unused solution.
A small air bubble may occur in Jext. It has no influence on either the use or the efficacy of the product.
Educational materials regarding the correct use, storage and care of Jext are available to prescribers, patients and caregivers including a Jext Trainer pen without needle or adrenaline for practising or instructing others in the correct use of Jext.
Note: the yellow cap prevents the device from activating, and should not be removed before injection is required. The black injector tip should be kept away from the hand.
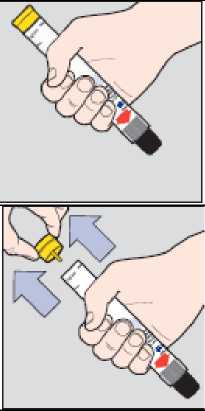
1. Grasp the Jext injector in your dominant hand (the one you use to write with) with your thumb closest to the yellow cap.
2. Pull off the yellow cap with your other hand.
|
3. Place the black injector tip against your outer thigh, holding the injector at a right angle (approx 90°) to the thigh. | ||
|
/nns ■_V ( IU, |
4. Push the black tip firmly into your outer thigh until you hear a ‘click’ confirming the injection has started, then keep it pushed in. Hold the injector firmly in place against the thigh for 10 seconds (a slow count to 10) then remove. The black tip will extend automatically and hide the needle. | |
|
/ J A" \ |
5. Massage the injection area for 10 seconds. Seek immediate medical help. Dial 999, ask for ambulance, state anaphylaxis. |
See Section 4.2 for instructions to be conveyed to the patient/carer regarding actions to be taken following each use of Jext.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Check the solution periodically through the viewing window of the unit to make sure the solution is clear and colourless.
Replace and discard the pre-filled pen if the solution is discoloured or contains a precipitate, or at the latest before the expiry date.
The expiry date is indicated on the label and Jext should not be used after this date.
7 MARKETING AUTHORISATION HOLDER
ALK-Abello A/S Boge Alle 6-8 DK-2970 Horsholm
8 MARKETING AUTHORISATION NUMBER(S)
PL 10085/0053
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 12 October 2010 Date of renewal of the authorisation: 26 June 2015
10 DATE OF REVISION OF THE TEXT
02/09/2016