Kabiven Emulsion For Infusion
FRESENIUS
KABI
PACKAGE LEAFLET: INFORMATION FOR THE USER
Kabiven™
emulsion for infusion
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, nurse or pharmacist
In this leaflet:
1. What is Kabiven and what it is used for
2. Before you receive Kabiven
3. How you are given Kabiven
4. Possible side effects
5. How Kabiven is stored
6. Further information
1. WHAT IS KABIVEN AND WHAT IT IS USED FOR
Kabiven comes in a three chamber bag in an overpouch. Kabiven contains the following medicines: amino acids (components used to build proteins), fat, glucose and electrolyte solutions. It provides energy (as sugar and fat) and amino acids (proteins) into your bloodstream when you cannot eat normally.
It is used as part of a balanced intravenous diet, together with salts, trace elements and vitamins which together provide your complete nutritional needs.
The full name of this medicine is Kabiven Emulsion for Infusion. It will be referred to as Kabiven throughout this leaflet.
2. BEFORE YOU RECEIVE KABIVEN
You should not receive Kabiven if you:
• have ever had an allergic reaction to Kabiven or any of the ingredients of Kabiven mentioned in section 6 (for symptoms of an allergic reaction please refer to section 4)
• are allergic to products containing egg, soya or peanut
• have too many fatty substances (like cholesterol) in your blood
• have seriously reduced liver function
• suffer from acute shock (resulting from heavy blood loss or allergic reaction)
• have a bleeding disorder associated with a condition known as haemophagocytotic syndrome or if your blood is not clotting properly.
• have a condition where your body has problems using proteins or amino acids
• have severe problems with your kidneys
• have hyperglycaemia (too much sugar in your blood) where the administration of more than 6 units of insulin per hour is required
• have raised levels of electrolytes (salts) in your blood
• have metabolic acidosis (the acid levels of your body fluids and tissues become too high)
• have too much fluid in your body - hyperhydration
• have fluid on your lungs (acute pulmonary oedema)
• are in a coma
• have heart problems
• are dehydrated with low levels of salts
• have severe sepsis (a condition in which your body is fighting a severe infection)
Care should be taken when administering Kabiven
Inform your doctor if you suffer from:
• reduced liver function
• untreated diabetes
• a condition where your body has problems using fat properly
• kidney problems
• any pancreas problems
• thyroid problems - hypothyroidism
• sepsis (a condition in which your body is fighting an infection)
• your body has problems eliminating electrolytes
• a condition where there is not enough oxygen in your body cells
• dehydration, as not drinking enough water causes the concentration of chemicals in your blood to increase (increased serum osmolarity).
This medicine may affect the results of other tests you may have. It is important to tell any doctor doing tests that you are using Kabiven.
Your doctor may want to do regular blood tests to make sure that the treatment with Kabiven is working correctly
Taking other medicines
Please tell your doctor or nurse if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Inform your doctor if you are taking
• a drug known as heparin which is used to prevent the formation and aid in the dispersion of blood clots
• warfarin, because Vitamin K|, which is contained in soybean oil, could affect the blood clotting ability
• Insulin for the treatment of diabetes
Pregnancy and breast-feeding
The safety of using Kabiven while pregnant or breast-feeding has not been looked into. If feeding directly into your vein (parenteral nutrition) becomes necessary during pregnancy or breast-feeding, your doctor will give you Kabiven only after careful consideration.
3. HOW YOU ARE GIVEN KABIVEN
You will receive your medicine by infusion only into a central vein. The dose of Kabiven and which bag size is used depends on your bodyweight in kilograms and your body's ability to use fat and sugar. Kabiven will be infused slowly over a period of 12-24 hours. Your doctor will decide on the correct dose for you or your child to receive. You may be monitored during your treatment.
Kabiven is not suitable for use in newborns or children under two years of age.
If you receive too much Kabiven
It is very unlikely that you will receive more infusion than you should as your doctor or nurse will monitor you during the treatment. The effects of an overdose may include nausea, vomiting, sweating and fluid retention.
Hyperglycaemia (too much sugar in your blood) and electrolyte disturbances have also been reported.
In case of overdose there is a risk of taking in too much fat. This is called 'fat overload syndrome'. See section 4 "Possible side effects" for more information. If you experience any of the symptoms described above or believe that you have received too much Kabiven inform your doctor or nurse immediately. The infusion may either be stopped immediately or continued with a reduced dosage. These symptoms will usually disappear on reducing the rate or stopping the infusion.
If you have any further questions on the use of this product, ask your doctor, nurse or pharmacist
4. POSSIBLE SIDE EFFECTS
Like all medicines, Kabiven can cause side effects, although not everybody gets them.
Very rarely (occurs in the less than 1 in 10000 patients) Kabiven may cause an allergic reaction. Tell your doctor immediately if:
• a bumpy and itchy rash appears on your body
• you have very high temperature
• you have difficulties breathing
• you get a fever
• you experience shivering
Common side effects (occurs in more than 1 in 100 patients)
• a slightly raised body temperature
The following uncommon side effects (occurs in less than 1 in 100 patients but in more than 1 in 1000 patients) have been observed
• chills and shivers
• tiredness
• stomach pain
• headache
• feeling sick or being sick
• increase of liver enzymes. Your doctor will tell you if this happens.
Other side effects are very rare (occurs in less than 1 in 10000 patients)
• high or low blood pressure
• difficulty in breathing
• prolonged, painful erections in men
• problems with your blood
Fat overload syndrome
This might happen when your body has problems using fat, because of having too much Kabiven. It may also happen because of a sudden change in your condition (such as kidney problems or infection). Possible symptoms are fever, increased levels of fat in your blood, your cells and your tissues, disorders in various organs and coma. All these symptoms will usually disappear if the infusion is discontinued.
If any of the side effects becomes serious or if you notice a side effect not listed in this leaflet, please tell your doctor, nurse or pharmacist.
5. HOW KABIVEN IS STORED
Keep out of the reach and sight of children.
Your doctor and hospital pharmacist are responsible for the correct storage, use and disposal of Kabiven. Do not store above 25° C. Do not freeze and always keep the container in the outer container.
The following Information Is intended for medical or healthcare professionals only:
Warnings and precautions for use
To avoid risks associated with too rapid infusion rates, it is recommended to use a continuous and well-controlled infusion, if possible by using a volumetric pump.
Since an increased risk of infection is associated with the use of any central vein, strict aseptic precautions should be taken to avoid any contamination especially during catheter insertion.
Serum glucose, electrolytes and osmolarity as well as fluid balance, acid-base status and liver and enzyme tests should be monitored.
Any sign or symptom of anaphylactic reaction (such as fever, shivering, rash or dyspnoea) should lead to immediate interruption of the infusion.
Kabiven should not be given simultaneously with blood in the same infusion set due to the risk of pseudoagglutination.
Method of administration
Intravenous use, infusion into a central vein.
To provide total parenteral nutrition, trace elements, vitamins and possibly electrolytes (taking into account the electrolytes already present in Kabiven) should be added to Kabiven according to the patients need.
Infusion rate
The maximum infusion rate for glucose is 0.25 g/kg/h.
Amino acid dosage should not exceed 0.1 g/kg/h.
Fat dosage should not provide more than 0.15 g/kg/h.
The infusion rate should not exceed 2.6 ml/kg body weight/hour (corresponding to 0.25 g glucose, 0.09 g amino acid and 0.1 g fat/ kg body weight). The recommended infusion period is 12-24 hours.
Precautions for disposal
Do not use if package is damaged. Use only if the amino acid and glucose solutions are clear and colourless or slightly yellow and the fat emulsion is white and homogenous. The contents of the three separate chambers have to be mixed before use and before any additions are made via the additive port.
After separation of the peelable seals the bag should be inverted three times to ensure a homogenous mixture which does not show any evidence of phase separation.
For single use only Any mixture remaining after infusion must be discarded.
Compatibility
Only medicinal or nutrition solutions for which compatibility has been documented may be added to Kabiven. Compatibility for different additives and the storage time of the different admixtures will be available upon request.
Additions should be made aseptically
Shelf-life
Shelf-life after mixing
After breaking the seals, chemical and physical in-use stability of the mixed three chamber bag has been demonstrated for 24 hours at 25°C.
Shelf-life after mixing with additives
After opening the peelable seals and mixing of the three solutions, additions can be made via additive port.
From a microbiological point of view the product should be used immediately when additions have been made. If not used immediately, the in-use storage time and conditions prior to use are the responsibility of the user and should normally not be longer than 24 hours at 2-8°C. If storage can not be avoided and provided that additions are made under controlled and validated aseptic conditions the mixed emulsion may be stored up to 6 days at 2-8°C before being used. After removal from storage at 2-8°C, the admixture should be infused within 24 hours.
Kabiven
Instructions for use
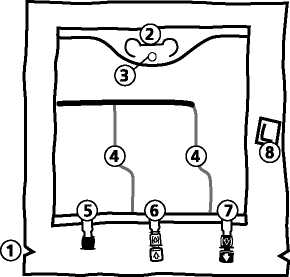
The bag
© Notches in the overpouch © Handle
® Hole for hanging the bag © Peelable seals
© Blind port (only used during Manufacturing) © Additive port ® Infusion port ® Oxygen absorber
1. Removal of overpouch
• To remove overpouch, hold the bag horizontally and tear from the notch close to the ports along the upper edge (A).
• Then simply tear the long side, pull off the overpouch V001
and discard it along with the oxygen absorber (B). xxx xxx
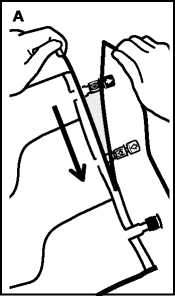
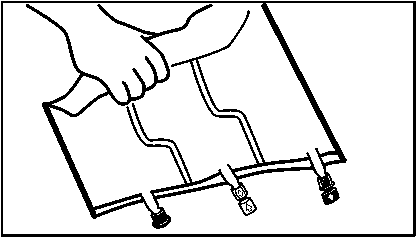
2. Mixing
A
3. Finalising the preparation:
• Place the bag on a flat surface.
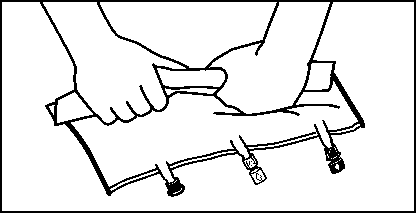
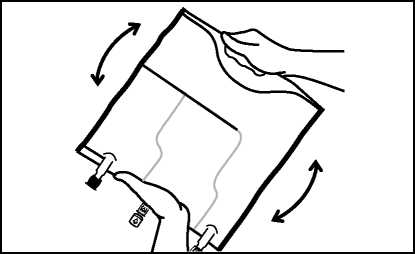
• Roll upthe bag tightly from the handle side towards the ports, firstly with the right hand and then applying a constant pressure with the left hand until the vertical seals are broken. The vertical peel seals open due to the pressure of the fluid. The peel seals can also be opened before removing the overpouch.
Please note: The liquids mix easily although the horizontal seal remains closed.
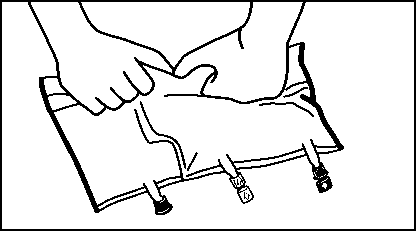
• Mix the contents of the three chambers by inverting the bag three times until the components are thoroughly mixed.
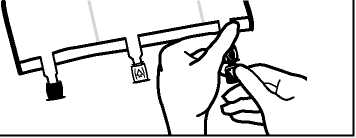
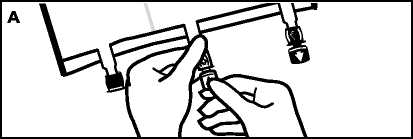
• Place the bag on a flat surface again. Shortly before injecting the additives, break off the tamper-evident arrow flag from the white additive port (A).
Please note: The membrane in the additive port is sterile.
• Hold the base of the additive port. Insert the needle, inject the additives (with known compatibility) through the centre of the injection site (B).
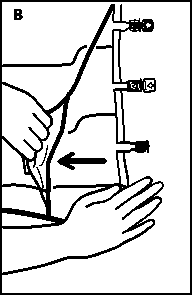
• Mix thoroughly between each addition by inverting the bag three times. Use syringes with needles of 18-25 gauge and a length of max. 40 mm.
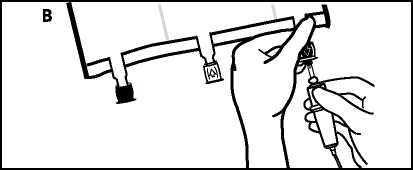
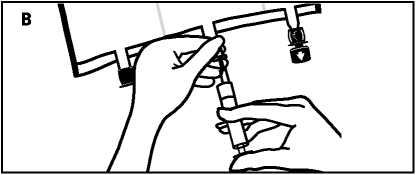
• Shortly before inserting the infusion set, break off the tamper evident arrow flag from the blue infusion port (A).
Please note: The membrane in the infusion port is sterile.
• Use a non-vented infusion set or close the air-inlet on a vented set.
• Hold the base of the infusion port.
• Push the spike through the infusion port.
The spike should be fully inserted to secure it in place. Please note: The inner part of the infusion port is sterile.
4. Hooking up the bag
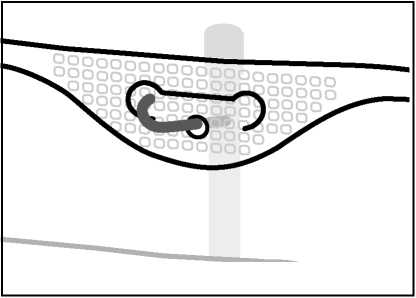
• Hook the bag up by the hole below the handle.
What Kabiven contains
Kabiven is available in a three chamber bag system. Each bag contains the following different volumes depending on the four pack sizes:
|
2566 ml |
2055 ml |
1540 ml |
1026 ml | |
|
Glucose (Glucose 19%) |
1516 ml |
1055 ml |
790 ml |
526 ml |
|
Amino acids and electrolytes (Vamin 18 Novum) |
750 ml |
600 ml |
450 ml |
500 ml |
|
Fat emulsion (Intralipid 20%) |
500 ml |
400 ml |
500 ml |
200 ml |
|
Active ingredients | ||||
|
Purified soybean oil |
100 g |
80 g |
60 g |
40 g |
|
Glucose monohydrate |
275 g |
220 g |
165 g |
110 g |
|
Corresponding to Glucose (anhydrous) |
250 g |
200 g |
150 g |
100 g |
|
Alanine |
12.0 g |
9.6 g |
7.2 g |
4.8 g |
|
Arginine |
8.5 g |
6.8 g |
5.1 g |
5.4 g |
|
Aspartic acid |
2.6 g |
2.0 g |
1.5 g |
1.0 g |
|
Glutamic acid |
4.2 g |
5.4 g |
2.5 g |
1.7 g |
|
Glycine |
5.9 g |
4.7 g |
5.6 g |
2.4 g |
|
Histidine |
5.1 g |
4.1 g |
5.1 g |
2.0 g |
|
Isoleucine |
4.2 g |
5.4 g |
2.5 g |
1.7 g |
|
Leucine |
5.9 g |
4.7 g |
5.6 g |
2.4 g |
|
Lysine hydrochloride |
8.5 g |
6.8 g |
5.1 g |
5.4 g |
|
Corresponding to Lysine |
6.8 g |
5.4 g |
4.1 g |
2.7 g |
|
Methionine |
4.2 g |
5.4 g |
2.5 g |
1.7 g |
|
Phenylalanine |
5.9 g |
4.7 g |
5.6 g |
2.4 g |
|
Proline |
5.1 g |
4.1 g |
5.1 g |
2.0 g |
|
Serine |
5.4 g |
2.7 g |
2.0 g |
1.4 g |
|
Threonine |
4.2 g |
5.4 g |
2.5 g |
1.7 g |
|
Tryptophan |
1.4 g |
1.1 g |
0.86 g |
0.57 g |
|
Tyrosine |
0.17 g |
0.14 g |
0.10 g |
0.07 g |
|
Valine |
5.5 g |
4.4 g |
5.5 g |
2.2 g |
|
Calcium chloride 2 H2O |
0.74 g |
0.59 g |
0.44 g |
0.29 g |
|
Corresponding to Calcium chloride |
0.56 g |
0.44 g |
0.55 g |
0.22 g |
|
Sodium glycerophosphate (anhydrous) |
5.8 g |
5.0 g |
2.5 g |
1.5 g |
|
Magnesium sulphate 7 H2O |
2.5 g |
2.0 g |
1.5 g |
0.99 g |
|
Corresponding to Magnesium sulphate |
1.2 g |
0.96 g |
0.72 g |
0.48 g |
|
Potassium chloride |
4.5 g |
5.6 g |
2.7 g |
1.8 g |
|
Sodium acetate 5 H2O |
6.1 g |
4.9 g |
5.7 g |
2.5 g |
|
Corresponding to Sodium acetate |
5.7 g |
2.9 g |
2.2 g |
1.5 g |
The emulsion must not be used after the expiry date shown on the label. The expiry date refers to the last day of that month. Do not use if the bag is leaking
For single use only. Any mixture remaining after infusion must be disposed of using the approved hospital procedures.
6. FURTHER INFORMATION
kabiven consists of a three chamber bag and an overpouch. An oxygen absorber is placed between the inner bag and the overpouch, which should be discarded before use. The inner bag is separated into three chambers by peelable seals.The contents of the three chambers have to be mixed before use, by opening the peelable seals.
Pack sizes:
1 X 1026 ml, 4 X 1026 ml 1 X 1540 ml, 4 X 1540 ml
1 x 2055 ml, 2 x 2055 ml (Excel), 4 x 2055 ml (Biofine)
1 x 2566 ml, 2 x 2566 ml (Excel), 5 x 2566 (Biofine)
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Fresenius kabi Ltd.,
Cestrian Court, Eastgate Way,
Manor Park, Runcorn, Cheshire,
WA7 1NT, U.k.
Manufacturer
Fresenius kabi AB,
SE-751 74 Uppsala, Sweden.
Fresenius kabi Austria GmbFI,
Flafnerstrasse 56,
AT-8055 Graz, Austria.
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Belgium |
kabiven | |
|
Denmark |
kabiven | |
|
Finland |
kabiven | |
|
France |
kabiven | |
|
Germany |
kabiven | |
|
Greece |
kabiven | |
|
Iceland |
kabiven | |
|
Ireland |
kabiven | |
|
Italy |
kabiven | |
|
Luxemburg |
kabiven | |
|
Netherlands |
kabiven | |
|
Portugal |
kabiven | |
|
Spain |
kabiven | |
|
Sweden |
kabiven | |
|
United kingdom |
kabiven |
This leaflet was last approved in December 2009
The other ingredients are purified egg phospholipids, glycerol, sodium hydroxide, glacial acetic acid and water for injections.
What Kabiven looks like and contents of the pack
V001
XXX XXX
Glucose and amino acid solutions are clear and colourless or slightly yellow and the fat emulsion is white.