Kalilactasol Solution For Haemofiltration And Haemodialysis
solution for haemofiltration and Package Leaflet: Information for the user
haemodialysis (UK)
Sodium chloride, calcium chloride dihydrate, magnesium chloride hexa-hydrate, potassium chloride, sodium lactate solution 60% w/w, glucose anhydrous.
Read all of this leaflet carefully before you start using this medicine.
• Seep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. WHAT KALILAcTAsoL Is AND WHAT It Is used For
2. before you are given kalilactasol
3. how to use kalilactasol
4. possible side effects
5. how to store kalilactasol
6. further information
1. WHAT kalilactasol is AND WHAT IT is UsED For
Salilactasol is used in hospitals in intensive care treatments called Continuous Renal Replacement Therapy. It is administered to correct any fluid and chemical imbalance of the blood, which is a consequence of kidney (renal) failure.
The treatments, using continuous renal replacement therapy, are designed to remove accumulated waste products from the blood when the kidneys are not functioning as they should.
The Kalilactasol solution is particularly used to treat critically ill patients with acute renal failure having a high concentration of potassium in the blood (hyperkalaemia).
Kalilactasol may also be used in cases of drug poisoning with dia-lysable or filterable substances.
2. before you are given kalilactasol
Do not use Kalilactasol during any of the following three conditions:
• if you have a low concentration of potassium in the blood (hypoka-laemia)
• if you suffer from a very severe metabolic problem with too low pH in your blood (acidosis)
• if you have difficulties in metabolising lactate, an acidic component which should be converted into bicarbonate and used to increase the pH of your blood.
Take special care with Kalilactasol
Before and during treatment, your blood condition will be checked.
For example, your acid-base balance and concentrations of salts (electrolytes) will be monitored.
Special attention should be given to:
• the level of potassium in your blood to ensure the most appropriate potassium concentration.
• the level of glucose in your blood, especially if you suffer from diabetes
You must tell your doctor if you are suffering from:
• liver disease
• heart disease
• general Infection of your blood (sepsis)
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
This is because the concentration in your blood of some of these medicines may be reduced during treatment with Salilactasol.
Your doctor will decide if other medicines should be changed.
If you are treated with digitalis due to heart problem the correction of the electrolyte concentration in your blood may lead to symptoms of digitalis overload.
This is especially the case if your blood level of digitalis is higher than normally intended.
Pregnancy and breast-feeding
There are no adequate data from the use of Salilactasol in pregnant or lactating women. Your doctor will decide whether you should be given Salilactasol if you are pregnant or breast-feeding.
3. how to use kalilactasol
Salilactasol is a sterile solution to be used in hospitals and administered by medical professionals only. The volume (which corresponds to the dose) of Salilactasol will depend on your condition.
The dose volume will be determined by the physician, who is responsible for your treatment.
Salilactasol can be administered into the venous blood line to replace fluid volume and electrolytes lost during the process of continuous haemofiltration or haemo-diafiltration (specific methods of Continuous Renal Replacement Therapies).
It can also be administered during continuous haemodialysis, where the sterile solution flows on one side of a dialysis membrane while the blood flows on the other side.
If you are given more Kalilactasol than you should be given:
Your fluid balance and blood chemistry will be carefully monitored. Therefore, it is unlikely that you
Kalilactasol solution contains glucose anhydrous 1.1 g/l and in
will be given more Kalilactasol than you should be given.
In the unlikely event that an overdose occurs, your doctor will take the necessary corrective measures and adjust your dose.
Overdose may result in electrolyte or acid-based disturbances as well as fluid overload if you suffer from renal failure. Overdose could also lead to problems with the heart.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. possible side effects
Like all medicines, Kalilactasol can cause side effects, although not everybody gets them.
The following side effects related to the use of Kalilactasol are possible:
• changes in levels of salt in your blood (electrolyte disturbances)
• high concentration of glucose in your blood (hyperglycaemia)
There are also some side effects which can be caused by the process of haemodialysis and haemofiltration, such as:
• nausea (feeling sick)
• vomiting (being sick)
• muscle cramps
• convulsions (fits)
• low blood pressure (hypotension)
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. how to store kalilactasol
Keep out of the reach and sight of children.
Do not use Kalilactasol after the expiry date which is stated on the label and the packaging. The expiry date refers to the last day of that month.
Do not store below +4 °C.
Do not use Kalilactasol if the solution is cloudy or if the over wrap is damaged. All seals must be intact. Kalilactasol can be disposed of via wastewater without harming the environment.
6. further information
What Kalilactasol contains The active substances are:
1000 ml of solution contains:
Calcium chloride
Magnesium chloride (hexahydrate) 0.152 g
Sodium lactate solution 60% w/w 7.471 g
(corresponding to sodium lactate anhydrous 4.483 g)
Glucose anhydrous 1.100 g
(in glucose monohydrate 1.210 g) mmol per litre:
Theoretical osmolarity:
301.6 mOsml/l
The other ingredients in Kalilactasol are:
• hydrochloric acid 3.646% w/v (for pH adjustment)
• water for injections
What Kalilactasol looks like and contents of the pack
Kalilactasol is presented in a one compartment bag. The solution is clear with a slightly yellow colour.
Each bag contains 5000 ml solution for haemofiltration and haemodialysis. The bag is over wrapped with a transparent film.
Each box contains two bags and one package leaflet.
Marketing Authorisation Holder:
Gambro Lundia AB,
Box 10 101,
SE-220 10 Lund, SWEDEN
Manufacturer:
Gambro Dasco S.p.A. Sondalo Plant, Via Stelvio 94,
IT-23035 Sondalo (SO), ITALY
This medicinal product is authorised in the Member states of the EEA under the following names:
Austria, Germany, Ireland, Portugal, United Kingdom: Kalilactasol Greece: Hemosol LG2
This leaflet was last approved in 03/2010
The following information is intended for medical or healthcare professionals only
Kalilactasol®
Solution for haemofiltration and haemodialysis
The solution is to be used only by or under the direction of a physician who should have sound experience of intensive care nursing and/or applied Continuous Renal Replacement Therapies such as haemofiltration, haemodiafiltration and haemodialysis.
The patient's haemodynamic, fluid, electrolyte and acid-base balance shall be closely monitored throughout the procedure. Close monitoring of the potassium levels must be carried out to enable selection of the most appropriate potassium concentration.
Severe metabolic acidosis shall be corrected with a bicarbonate solution prior to using a lactate based substitution fluid.
When used with a monitor, only monitors for Continuous Renal Replacement Therapy must be used.
The volume of Kalilactasol to be administered will depend on the patient's clinical condition and the target fluid balance. Continued application of haemofiltration, haemo-diafiltration or haemodialysis will remove excess fluid and electrolytes.
Do not mix with bicarbonate containing solutions, as this may cause precipitation of calcium and magnesium carbonate.
Kalilactasol, when used as a substitution solution, is administered into the extracorporeal blood circuit before (pre-dilution) or after (post-dilution) the hemofilter.
In continuous haemodialysis or haemofiltration, the clearances obtained are directly proportional to the dialysate flow.
The range of flow rates used for the substitution solution in haemofiltration and haemodiafiltration are:
• Adult: 500 - 1500 ml/hour
• Children: 15-20 ml/kg/hour
The range of flow rates for the dialysis solution in haemofiltration or continuous haemodialysis are:
• Adult: 500 - 2000 ml/hour
• Children: 15-20 ml/kg/hour
INSTRUCTIONS FOR USE / HANDLING
Aseptic technique shall be used throughout the administration to the patient.
Use only if the solution is clear and the over wrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
If heating of the solution to body temperature (37 °C) is necessary the procedure must be carefully controlled verifying that the solution is clear and without particles.
The bag is fitted with an injection port for the possible addition of other necessary drugs. Medication shall only be added to the solution under the responsibility of a physician in the following way:
Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. the solution must be administered immediately.
The dialysis or replacement fluid line may be connected to either of the two access ports.
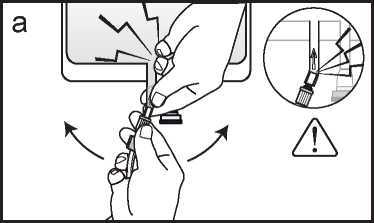
a If the luer access is used, remove the cap and connect the male luer lock on the dialysis or replacement fluid line to the female luer connector on the bag; tighten. Using both hands, break the frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure a below)
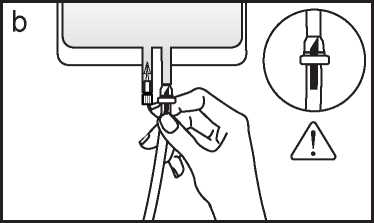
b If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure b below)
The solution is for immediate and single use only. Discard any unused solution immediately after use.
D0xxxxxxx Rev. 2009-10/2
Kalilactasol PL GB REG.indd 3 2009-10-13 11:18:51