Klaricid Iv 500 Mg

CD
O
oo
un
° INFORMATION FOR THE HEALTHCARE
« PROFESSIONAL
KLARICID1* I.V. 500 mg 500 mg clarithromycin
Refer to the Summary of Product Characteristics for the full prescribing information
Method of administration
Refer to the summary of product characteristics for posology information.
Clarithromycin should not be given as a bolus or an intramuscular injection. Klaricid IV should be administered into one of the larger proximal veins as an IV infusion over 60 minutes, using a solution concentration of about 2mg/ml.
STEP 1 STEP 2
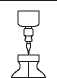
10 ml

Add 10 ml sterilised Water for Injections into the vial and shake Use within 24 hours May be stored from 5°C up to room temperature
Add 10ml from Step 1 to 250ml of a suitable diluent (see below)
This provides a 2mg/ml solution
Use within 6 hours (at room temperature) or within 24 hours if stored at 5°C
Recommended Diluents
5% dextrose in Lactated Ringer's Solution,
5% dextrose, Lactated Ringer’s solution, 5% dextrose in 0.3% sodium chloride, Normosol-M in 5% dextrose, Normosol-R in 5% dextrose, 5% dextrose in 0.45% sodium chloride, or 0.9% sodium chloride. Compatibility with other IV additives has not been established.
Storage: Do not store above 30°C. Store in the original container as the powder is sensitive to light. See carton and vial for expiry date. The product should not be used after this date.
Product Licence Number: 46302/0018 Legal Category: POM
Marketing Authorisation Holder - Mylan Products Ltd., 20 Station Close, Potters Bar, Herts, EN6 1TL, UK
Revised: June 2016
PATIENT INFORMATION LEAFLET ON
KLARSCSD^N 500 mg
500mg clarithromycin
Read all of this leaflet carefully before
you start taking this medicine because it
contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in the leaflet. See section 4.
What is in this leaflet:
1. What Klaricid IV is and what it is used for
2. What you need to know before receiving Klaricid IV
3. How is Klaricid IV given?
4. Possible side effects
5. How to store Klaricid IV
6. Contents of the pack and other information
1. What Klaricid IV is and what it is used for
DO NOT USE
• Diluents containing preservatives
• Diluents containing inorganic salts
DO NOT USE
• Solution strengths greater than 2mg/ml {0.2%)
• Rapid infusion rates (< 60 minutes)
• Failure to observe these precautions may result in pain along the vein
Klaricid IV contains the active ingredient clarithromycin. Klaricid belongs to a group of medicines called macrolide antibiotics. Antibiotics stop the growth of bacteria (bugs) that cause infections.
Klaricid IV is used whenever an intravenous (injection into the vein) antibiotic is required to treat severe infections or, alternatively, if a patient cannot swallow Klaricid tablets.
It is used to treat infections such as:
1. Chest infections such as bronchitis and pneumonia
2. Throat and sinus infections
3. Skin and tissue infections
Klaricid IV is indicated in adults and children 12 years and older.
2. What you need to know before you receive Klaricid IV
Do not receive Klaricid IV if you;
• know that you are allergic to clarithromycin, other macrolide antibiotics such as erythromycin or azithromycin, or any of the other ingredients in Klaricid IV.
• are taking medicines called ergotamine or dihydroergotamine tablets or use ergotamine inhalers for migraine.
• are taking medicines called terfenadine or astemizole (widely taken for hay fever or allergies) or cisapride (for stomach disorders) or pimozide (for mental health problems) as combining these drugs can sometimes cause serious disturbances in heart rhythm.
• are taking lovastatin or simvastatin (HMG-CoA reductase inhibitors, commonly known as statins, used to lower levels of cholesterol (a type of fat) in the blood).
• have low levels of potassium in the blood (a condition known as hypokalaemia).
• have severe liver disease with kidney disease.
• have an irregular heart rhythm.
• are taking medicines called ticagrelor or ranolazine (for heart attack, chest pain or angina).
• are taking colchicine (usually taken for gout)
Klaricid IV is not suitable for use in children
under 12 years of age.

Warnings and precautions
Talk to your doctor or pharmacist before being given Klaricid IV;
• if you have any liver or kidney problems
• if you have, or are prone to, fungal infections (e.g. thrush)
• if you are pregnant or breast feeding Other medicines and Klaricid IV
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, as your dose may need to be changed or you may need to have regular tests performed:
• digoxin, quinidine or disopyramide (for heart problems)
• warfarin (for thinning the blood)
• carbamazepine, valproate, phenobarbital or phenytoin (for epilepsy)
• atorvastatin, rosuvastatin (HMG-CoA reductase inhibitors, commonly known as statins, and used to lower levels of cholesterol (a type of fat) in the blood). Statins can cause rhabdomyolosis (a condition which causes the breakdown of muscle tissue which can result in kidney damage) and signs of myopathy (muscle pain or muscle weakness) should be monitored.
• nateglinide, pioglitazone, repaglinide, rosiglitazone or insulin (used to lower blood glucose levels)
• theophylline (used in patients with breathing difficulties such as asthma)
• triazolam, alprazolam or midazolam (sedatives)
• cilostazol (for poor circulation)
• omeprazole (for treatment of indigestion and gastric ulcers)
• methylprednisolone (a corticosteroid)
• vinblastine (for treatment of cancer)
• ciclosporin, sirolimus and tacrolimus (immune suppressants)
• etravirine, efavirenz, nevirapine, ritonavir, zidovudine, atazanavir, saquinavir (anti- viral drugs used in the treatment of HIV)
• rifabutin, rifampicin, rifapentine, fluconazole, itraconazole (used in the treatment of certain bacterial infections)
• tolterodine (for overactive bladder)
• verapamil, amlodipine, diltiazem (for high blood pressure)
• sildenafil, vardenafil and tadalafil (for impotence in adult males or for use in pulmonary arterial hypertension (high blood pressure in the blood vessels of the lung))
• St John’s Wort (a herbal product used to treat depression)
• aminoglycosides (a group of antibiotic to treat certain bacteria for example gentamicin, neomycin) and other medicines that may be toxic to the ear.
Klaricid IV does not interact with oral contraceptives.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist before receiving Klaricid IV as the safety of clarithromycin in pregnancy or breast-feeding is not known.
Driving and Using Machines:
Klaricid IV may make you feel dizzy or drowsy. If they affect you in this way do not drive, operate machinery or do anything that requires you to be alert.
3. How is Klaricid IV given?
Klaricid IV is prepared by your doctor or nurse by dissolving the powder in the vial in sterile water. The solution obtained is added to a
31 01518 06_d1
FRONT

|
5. How to store Klaricid IV | |
|
Keep out of the sight and reach | |
|
f'itJRK |
of children |
|
Do not use after the expiry date on | |
|
the carton and vial. | |
|
p-JESiy |
> Do not store above 30°C. Store |
|
I ~ Ul |
in the original container. The |
|
L ixx |
reconstituted solution will be |
|
stored for either 6 hours at room | |
|
temperature or for 24 hours at | |
|
5° - 25°C. | |
|
6. Content of the pack and other | |
|
information | |
larger volume of sterile liquid. Klaricid IV is given to you slowly through a needle, into your vein over a period of at least an hour.
The usual dose of Klaricid IV for adults and children over 12 years is 1 .Og per day, split into two doses, for 2 to 5 days. Your doctor will work out the correct dose for you.
Children under 12 years should not be given Klaricid IV. Your doctor will prescribe another suitable medicine for your child.
If a child accidentally swallows some of this medicine, seek medical advice urgently.
If you are given more Klaricid IV than you should have
As Klaricid IV is given to you by a doctor, an overdose is unlikely but symptoms may include vomiting and stomach pains.
4. Possible side effects
Like all medicines, Klaricid IV can cause side effects although not everybody gets them.
If you suffer from any of the following at any time during your
treatment tell your doctor immediately as your
treatment may need to be stopped:
• severe or prolonged diarrhoea, which may have blood or mucus in it. Diarrhoea may occur over two months after treatment with clarithromycin, in which case you should still contact your doctor.
• a rash, difficulty breathing, fainting or swelling of the face and throat. This is a sign that you may have developed an allergic reaction.
• yellowing of the skin (jaundice), skin irritation, pale stools, dark urine, tender abdomen or loss of appetite. These may be signs that your liver may not be working properly.
• severe skin reactions such as blistering of the skin, mouth, lips, eyes and genitals (symptoms of a rare allergic reaction called Stevens-Johnson syndrome/toxic epidermal necrolysis).
• muscle pain or weakness known as rhabdomyolysis (a condition which causes the breakdown of muscle tissue which can result in kidney damage).
Common side effects of Klaricid IV include;
• inflammation, tenderness or pain at the site of the injection
• headache
• difficulty sleeping
• changes in sense of taste
• stomach problems such as feeling sick, vomiting, stomach pain, indigestion, diarrhoea
• a change in the way your liver works
• skin rash
• increased sweating
Other less common side effects include:
• swelling, redness or itchiness of the skin
• acne
• asthma
• oral or vaginal ‘thrush’ (a fungal infection)
• reduction in the level of certain blood cells (which can make infections more likely or increase the risk of bruising or bleeding)
• loss of appetite, heartburn, bloating, constipation, wind
• inflammation of the pancreas
• anxiety, nervousness, drowsiness, tiredness, dizziness, tremor or shaking
• confusion, loss of bearings, hallucinations (seeing things), change in sense of reality or panicking, depression, abnormal dreams or nightmares
• convulsion (fits) or loss of consciousness
• ringing in the ears or hearing loss
• vertigo
• paraesthesia, more commonly known as ‘pins and needles'
• nose bleeds
• leaking of blood from blood vessels (haemorrhage)
• inflammation of the mouth or tongue
• discolouration of the tongue or teeth
• dry mouth
• loss of taste or smell or inability to smell properly
• joint pain
• muscle pain or loss of muscle tissue. If you suffer from myasthenia gravis (a condition in which the muscles become weak and tire easily) or rhabdomyolysis (a condition which causes the breakdown of muscle tissue), clarithromycin may worsen these symptoms
• heart attack, chest pain or changes in heart rhythm such as palpitations
• a change in the levels of products made by the liver, inflammation of the liver or an inability of the liver to function property (you may notice yellowing of the skin, dark urine, pale stools or itchiness of the skin)
• a change in the levels of products produced by the kidney, inflammation of the kidney or an inability of the kidney to function property (you may notice tiredness, swelling or puffiness in the face, abdomen, thighs or ankles or problems with urination)
• a change in the levels of certain cells or products found in the blood
Reporting side effects
If you get any side effects, talk to your doctor
or pharmacist. This includes any possible side
effects not listed in this leaflet.
What Klaricid IV contains
Klaricid IV contains the active ingredient clarithromycin.
The other ingredients are; lactobionic acid, sodium hydroxide, and nitrogen.
This medicinal product contains less than 23mg sodium (1 mmol) per 500mg i.e. essentially “sodium-free”.
What Klaricid IV looks like and contents of the pack
Klaricid IV is available in vials containing 500mg of clarithromycin as the active ingredient. When made up with Water for Injections, each millilitre (ml) of solution contains 2mg of clarithromycin.
Marketing Authorisation Holder and Manufacturer:
Marketing Authorisation Holder - Mylan Products Ltd., 20 Station Close, Potters Bar, Herts, EN6 1TL, UK Manufacturer - FAMAR L’Aigle Usine St Remy, Rue de L’lsle, 28380 Saint Remy sur Avre, France.
This leaflet was last [fjfj
revised in June 2016 HSU lv,y|an
31 01518 06
BACK
31 01518 06_d1