Kolneb 2 Miu. Powder For Nebuliser Solution
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Kolneb 2 MIU., Powder for Nebuliser Solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 10 ml vial contains 2 million I.U. (MIU) colistimethate sodium (CMS). For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder for nebuliser solution.
White powder.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Kolneb 2 MIU. is indicated for the treatment by nebulisation of colonisation and infections of the lung due to susceptible Pseudomonas aeruginosa in patients with cystic fibrosis.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
4.2 Posology and method of administration
Sputum cultures should be obtained to confirm colonisation with Pseudomonas aeruginosa sensitive to colistimethate sodium prior to initiating treatment with Kolneb 2 MIU.
Posology
The following information provides guidance on recommended doses and the dose should be adjusted according to clinical response:
Adults:
1 or 2 MIU
twice or three times daily
Paediatric population
Children (2 - 11 years) and adolescents (12 - 17 years): 1 or 2 MIU
twice or three times daily
Children < 2 years: 0.5 -
1 MIU twice daily
In premature and newborn infants special care should be employed as renal function is only insufficiently developed in this population.
The dosage is determined by the severity and type of infection.
The dose may be varied across this range depending on the condition being treated.
Initial colonisation with Pseudomonas aeruginosa sensitive to colistimethate sodium may be treated with a 3 week course of 2 MIU twice daily in conjunction with other parenteral or oral antibiotics.
For frequent, recurrent infections (less than three positive cultures of Pseudomonas aeruginosa sensitive to colistimethate sodium in a six-month period) the dose may be increased up to a maximum of 2 MIU three times daily for up to 3 months, in conjunction with other parenteral or oral antibiotics.
Chronic colonisation (three or more positive cultures of Pseudomonas aeruginosa sensitive to colistimethate sodium in a 6-month period) may require long term therapy with 1 to 2 MIU twice daily.
Additional parenteral or oral antibiotics may need to be administered to treat acute exacerbations of pulmonary infection.
Renal impairment
Colistimethate sodium is known to be excreted renally and therefore administration of Kolneb 2 MIU. in patients with renal failure should be undertaken with caution. There are however no data on this population treated with nebulized colistimethate sodium. Since the systemic concentration of colistin following inhaled administration of Kolneb 2 MIU. is low (see section 5.2), no dose adjustment in case of renal failure is recommended. However, appearance of neurotoxic reactions as well as renal function should be monitored.
Nebulised Kolneb 2 MIU. should be administered after physiotherapy and other inhaled treatments, where used. Other inhaled therapies may include agents to reduce the viscoelasticity of sputum and bronchodilators (see section 4.4).
Method of administration
Kolneb 2 MIU. is intended for administration by nebulisation using a suitable nebuliser.
Drug delivery characteristics from in vitro studies with different nebuliser systems are detailed below:
|
Nebuliser system |
PARI LC Plus |
PARI LC Sprint |
eFlow rapid |
|
Total Drug Delivered from Nebuliser mouthpiece [Million IU] |
1.325 |
1.389 |
1.106 |
|
Drug Delivery Rate [Million IU/minute] |
0.120 |
0.136 |
0.217 |
|
Fine Particle Fraction [% < 5 gm] |
51.3 |
60.1 |
48.1 |
|
Droplet Size Distribution/Mass Median Aerodynamic Diameter (MMAD) [gm] |
4.7 |
3.9 |
5.1 |
|
Geometric Standard Deviation (GSD) |
2.2 |
2.2 |
2.1 |
|
Measured using Kolneb 2 MIU. reconstituted with 4 ml of 0.9 sodium chloride solution | |||
160 mg colistimethate sodium correspond approximately to 2 MIU.
Colistimethate sodium is very soluble in the reconstitution medium. The recommended technique for dissolving the drug product is the addition of 4 ml of isotonic sodium chloride solution (0.9% w/w), to the vial containing Kolneb 2 MIU. followed by gentle shaking. Due to potential foaming, vigorous shaking should be avoided.
The resulting solution for nebulisation should be clear and carefully transferred into the medication reservoir of the nebuliser.
The solution is for single use only and any remaining solution should be discarded.
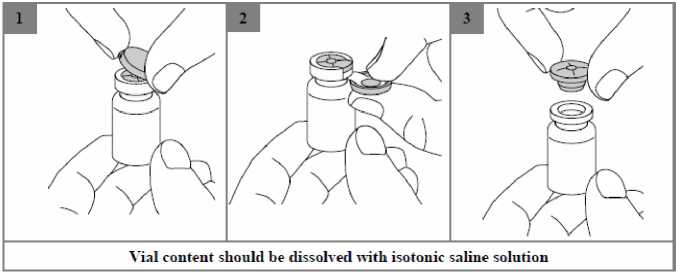
Suitable nebulisers are the reusable jet nebulisers PARI LC PLUS or PARI LC SPRINT, which are used with a suitable compressor, or the membrane nebuliser eFlow rapid.
• The nebuliser must be kept according to the instructions of use of the corresponding nebuliser during operation.
• The patient should sit in an upright position during inhalation. Inhalation should be performed applying a normal breathing pattern without interruption.
• The nebuliser must be cleaned and disinfected after use as described in the instructions of use of the corresponding nebuliser.
For special precautions for disposal and handling of reconstituted solutions,
see section 6.6.
4.3 Contraindications
Hypersensitivity to colistimethate sodium, colistin sulphate or to other polymyxins.
4.4 Special warnings and precautions for use
Kolneb 2 MIU. should be used with extreme caution in patients with myasthenia gravis because of the potential for drug induced neuromuscular blockade.
Nebulisation of colistimethate sodium may induce coughing or bronchospasm.
It is advisable to administer the first dose under medical supervision. Predosing with a bronchodilator is recommended and should be routine, especially if this is part of the patient's current therapeutic regimen.
FEV1 should be evaluated pre and post dosing.
If there is evidence of colistimethate sodium induced bronchial hyperreactivity in a patient not receiving pre-treatment bronchodilators the test should be repeated on a separate occasion using a bronchodilator. Evidence of bronchial hyperreactivity in the presence of a bronchodilator may indicate an allergic response and Kolneb 2 MIU. should be discontinued.
Bronchospasm that occurs should be treated as medically indicated.
Bronchial hyperreactivity in response to colistimethate sodium may develop with continued use over time and it is recommended that pre and post treatment FEVls are evaluated at regular clinic visits.
Renal impairment
Colistimethate sodium is renally excreted and is nephrotoxic if high serum concentrations are achieved. Whilst this is unlikely during inhalation therapy, appearance of neurotoxic reactions as well as the renal function should be monitored especially in patients with renal impairment.
Nephrotoxicity
Impairment of renal function has been reported, usually following use of higher than recommended intravenous or intramuscular doses in patients with normal renal function, or failure to reduce the intravenous or intramuscular dosage in patients with renal impairment or when used concomitantly with other nephrotoxic antibiotics. The effect is usually reversible on discontinuation of therapy.
Neurotoxicity
High serum concentrations of colistimethate sodium after intravenous or intramuscular administration, may be associated with overdosage or failure to reduce the dosage in patients with renal impairment, and this may lead to neurotoxicity. Concomitant use with either non-depolarising muscle relaxants or antibiotics with similar neurotoxic effects can also lead to neurotoxicity. Dose reduction of colistimethate sodium may relieve symptoms. Neurotoxic effects that have been reported include: vertigo, transient facial paraesthesia, slurred speech, vasomotor instability, visual disturbances, confusion, psychosis and apnoea (see also section 4.5).
Porphyria
Use with extreme caution in patients with porphyria.
Microbial Resistance
Colistimethate sodium acquired resistance in Pseudomonas aeruginosa during clinical use has been reported. Susceptibility testing should be performed on patients who are treated on a long term basis (see section 5.1).
4.5 Interaction with other medicinal products and other forms of interaction
Due to the effects of colistimethate sodium on the release of acetylcholine, nondepolarising muscle relaxants should be used with extreme caution in patients receiving Kolneb 2 MIU. as their effects could be prolonged (see section 4.4).
Concomitant use of colistimethate sodium with other medicinal products of neurotoxic and/or nephrotoxic potential (e.g. cephalosporins, aminoglycosides, nondepolarising muscle relaxants) including those which are administered by the i.v. or i.m. routes should only be undertaken with the greatest caution (see section 4.4).
4.6 Fertility, Pregnancy and lactation
Pregnancy
Safety in human pregnancy has not been established. Animal studies are insufficient with respect to the effect of colistimethate sodium on reproduction and development (see section 5.3). However there is evidence that colistimethate sodium crosses the placenta and consequently there is potential for foetal toxicity if administered during pregnancy. Kolneb 2 MIU. should only be given during pregnancy if the benefits outweigh any potential risk.
Breastfeeding
Colistimethate sodium is secreted in breast milk. Colistimethate sodium should be administered to breastfeeding women only when clearly indicated and the benefit to the mother outweighs the potential risk to the child.
4.7 Effects on ability to drive and use machines
Neurotoxicity, characterised by dizziness, confusion or visual disturbances has been reported following parenteral administration of colistimethate sodium. If these effects occur, patients should be warned not to drive or operate machinery.
4.8 Undesirable effects
The most common undesirable effects following nebulisation of colistimethate sodium are coughing and bronchospasm (indicated by chest tightness which may be detected by a decrease in FEV1) in approximately 10% of patients (see also section 4.4).
The likelihood of adverse events may be related to the age, renal function and condition of the patient.
Adverse reactions are tabulated below by system organ class and frequency. Frequencies are defined as:
Very common (>1/10); common (>1/100 to <1/10); uncommon (>1/1,000 to <1/100); rare
(>1/10,000 to <1/1,000), very rare (<1/10,000), not known (cannot be estimated from the available data).
|
Body System |
Frequency |
Reported adverse reaction |
|
Immune system disorders |
Not known |
Hypersensitivity reactions such as skin rash |
|
Respiratory, thoracic and mediastinal disorders |
Very common |
Cough, chest tightness, bronchoconstriction or bronchospasm |
|
General disorders and administration site conditions |
Not known |
Sore throat and sore mouth. |
Should hypersensitivity reactions such as skin rash occur treatment with colistimethate sodium should be withdrawn.
Cases of sore throat or sore mouth may be due to hypersensitivity or superinfection with Candida species.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions the Yellow Card Scheme (Website: www.mhra.gov.uk/yellowcard).
4.9 Overdose
Symptoms:
Overdosage may cause apnoea, muscle weakness, vertigo, transient facial paraesthesia, slurred speech, vasomotor instability, visual disturbances, confusion, psychosis and renal insufficiency.
Treatment:
No antidote is available. Management of overdose is by means of supportive treatment and measures designed to increase clearance of colistimethate sodium such as inducing an osmotic diuresis with mannitol or prolonged haemodialysis.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other antibacterials, polymyxins, ATC code: J01X B01.
Mechanism of action
Colistimethate sodium (also known as colistin) is a cyclic polypeptide antibiotic derived from Paenibacilluspolymyxa var. colistinus and belongs to the polymyxin group. The polymyxin antibiotics are cationic, surface active agents and act by binding to and changing the permeability of the bacterial cell membrane causing bacterial cell death. Polymyxins are bactericidal against Gram-negative bacteria with a hydrophobic outer membrane.
PK/PD relationship
Polymyxins have been reported to have a concentration-dependent bactericidal effect on susceptible bacteria.
Epidemiological cut-off values
The epidemiological cut off value for colistin for Pseudomonas aeruginosa, distinguishing the wild type population from isolates with acquired resistance traits, is 4 mg/l (EUCAST Database 2013).
Mechanisms of resistance
Resistance develops due to modifications of lipopolysaccharide (LPS) or other components in the bacterial cell membrane.
Cross resistance
There is no known co-resistance of colistin with other antibiotic classes.
5.2 Pharmacokinetic properties
Absorption
Gastrointestinal absorption is negligible hence the swallowing of colistimethate sodium deposited in the nasopharynx is unlikely to add to the systemic exposure.
Absorption following lung administration is influenced by the nebuliser system, aerosol droplet size and disease state of the lungs.
Pharmacokinetics
A study in healthy volunteers, who inhaled colistimethate sodium, demonstrated the Cmax of polymyxin E1 (the active moiety) varied between 40.0 and 69.9 ng/mL and the AUC varied between 350 and 668 ng/mL/h depending on the nebuliser and the fill volume and concentration, which varied the dose from 0.3 million IU to 2 million IU. The half-life was approximately 5.2 hours. The absolute bioavailability was calculated to vary between 5% and 18% depending on the nebuliser. The AUC following an intravenous dose of 0.5 million IU was 3,352 ng/mL/h and the Cmax was 1,232 ng/mL.
Biotransformation
Colistimethate sodium undergoes conversion to its base in vivo.
Elimination
There is no information on the elimination of colistimethate sodium following nebulisation.
Following i.v. administration excretion is primarily renal with 62% of a parenteral dose recovered in the urine within 8 hours and around 80% in 24 hours.
5.3 Preclinical safety data
Animal studies are insufficient with respect to effects on reproduction. There were no notable effects on fertility or general reproductive performance in male or female rats or mice.
Reproductive toxicity studies in rats and mice do not indicate teratogenic properties. However colistimethate sodium given intramuscularly during organogenesis to rabbits at 4.15 and 9.3 mg/kg resulted in talipes varus in 2.6 and 2.9% of foetuses respectively. These doses are 0.5 and 1.2 times the maximum daily human dose. In addition increased resorption occurred at 9.3 mg/kg.
Data on potential genotoxicity are limited and carcinogenicity data for colistimethate sodium are lacking. Colistimethate sodium has been shown to induce chromosomal aberrations in human lymphocytes, in vitro. This effect may be related to a reduction in mitotic index, which was also observed.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
None.
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
3 years
Reconstituted solutions:
Solutions can be stored after reconstitution with 0.9% saline solutions (4 ml for Kolneb 2 MIU.) for 24 hours below 25°C. Storage for more than 24 hours is not recommended due to the risk of microbial contamination of the reconstituted solution.
6.4 Special precautions for storage
Do not store above 25°C. Keep the vials in the outer carton.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5 Nature and contents of container
Kolneb 2 MIU.: 10 ml colourless glass vials with lilac flip-tear-off caps.
• 1 vial
• 2 vials
• 10 vials
• 14 vials
• 28 vials
• 56 vials
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Conventional nebulisers produce a continuous aerosol stream, which means that some of the nebulised Kolneb 2 MIU. may be released into the environment on exhalation. Kolneb 2 MIU. should therefore be administered in well ventilated rooms if conventional nebulisers are used. The use of special filters/valve sets can prevent the inhaled medicinal product from entering ambient air on exhalation.
The nebuliser must be cleaned and disinfected after each use in accordance with the manufacturer's instructions.
7 MARKETING AUTHORISATION HOLDER
YES Pharmaceutical Development Services GmbH Bahnstr. 42 - 46 61381 Friedrichsdorf Germany
8 MARKETING AUTHORISATION NUMBER(S)
PL 16866/0100
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
22/05/2014
10 DATE OF REVISION OF THE TEXT
22/05/2014