Konakion Mm Paediatric 2 Mg/0.2Ml
Konakion® MM <*>§>
Paediatric 2 mg/0.2 ml
solution for injection or oral administration
Phytomenadione (vitamin K^)
Please read all of this leaflet carefully before
your baby or child is given this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask the doctor, nurse or midwife.
• This medicine has been prescribed for your child. Do not pass it on to others. It may harm them even if their symptoms are the same.
• If any of the side effects become serious or troublesome, or if you notice any side effects not listed in this leaflet, please tell the doctor, nurse or midwife.
In this leaflet:
1. What Konakion MM Paediatric is and what it is used for
2. Before your baby or child is given Konakion MM Paediatric
3. How Konakion MM Paediatric is given
4. Possible side effects
5. How Konakion MM Paediatric is stored
6. Further information
1. What Konakion MM Paediatric is and what it is used for
Konakion MM Paediatric contains a medicine called phytomenadione. This is a man-made vitamin called vitamin K1. Konakion MM Paediatric is used for the following:
• Babies who do not have enough vitamin K in their bodies. Giving Konakion MM Paediatric prevents and treats bleeding caused by a lack of vitamin K. This is called ‘vitamin K deficiency bleeding’ (VKDB). This is a serious, but rare condition. All newborn babies are given vitamin K1 with their parent’s permission.
• Babies and young children who may have had too much of certain medicines to thin their blood (called anticoagulants). Konakion MM Paediatric is normally used to treat these children after advice from a specialist haematologist (blood doctor).
Konakion MM Paediatric works by helping your body make blood clotting factors. These blood clotting factors help stop bleeding.
2. Before your baby or child is given Konakion MM Paediatric
Your child must not be given Konakion MM Paediatric if they are allergic (hypersensitive) to:
• Phytomenadione or any of the other ingredients of Konakion MM Paediatric (listed in Section 6: Further information).
If you are not sure if this applies to your child, talk to the doctor, nurse or midwife before they are given Konakion MM Paediatric.
Take special care with Konakion MM Paediatric
Check with your doctor, nurse or midwife before your child has Konakion if:
• They have a problem with the flow of bile in their body (cholestatic disease). Bile is important in helping the body to use some vitamins.
Taking other medicines
Please tell your doctor, nurse or midwife if your child is taking or has recently taken any other medicines. This includes medicines that you buy without a prescription and herbal medicines.
This is because Konakion MM Paediatric can affect the way some medicines work. Also some other medicines may affect the way Konakion MM Paediatric works.
In particular, tell your doctor, nurse or midwife if your baby or child is taking medicines to stop their blood clotting (anticoagulants).
Important information about some of the ingredients of Konakion MM Paediatric
Konakion MM Paediatric is essentially ‘sodium free’ as it contains less than 1 millimole sodium (2.64 mg in each millilitre).
1
3. How Konakion MM Paediatric is given
Konakion MM Paediatric can be given to your child by injection into a vein or muscle or by mouth (orally). How it is given will depend upon what the medicine is being used for and whether your baby was born prematurely. The doctor will decide how much Konakion MM Paediatric your child needs.
Prevention of vitamin K deficiency bleeding
Healthy babies delivered at or nearly full term
These babies will be given either:
• A single injection (1 mg) either at birth or soon after, or
• By mouth (oral) a first dose (2 mg) at birth or soon after. This is followed by a second 2 mg dose after 4 to 7 days and third 2 mg dose at
1 month. In exclusively formula fed infants the third oral dose can be omitted.
Premature babies or full term babies at
special risk of bleeding
• These babies will be given Konakion MM Paediatric as an injection at birth or soon after.
• More injections may be given later if your baby is still at risk of bleeding.
Further doses:
• Babies who are given vitamin K by mouth and who are breast-fed (not given formula milk) may need more doses of vitamin K by mouth.
• Bottle-fed babies given the two doses of vitamin K by mouth may not need any more doses of vitamin K. This is because it is included in formula milk.
The instructions ‘How to give your baby
Konakion MM Paediatric by mouth’ are given
later in this section (section 3).
Treatment of vitamin K deficiency bleeding (VKDB)
• These babies will be given Konakion MM Paediatric as an injection (usually 1 mg).
• More injections may be given later if your baby is still at risk of bleeding. Some babies may also need a blood transfusion.
Treatment of too much blood thinning medicine
Treatment of children who have had too much blood thinning medicine is usually decided by a haematologist (blood doctor).
• Konakion Mm Paediatric will be given by injection into one of your child’s veins (IV injection).
• The doctor will usually check your child’s blood for the levels of clotting factors. This check will be made 2 to 6 hours after giving Konakion MM Paediatric.
• If your child still does not have enough blood clotting factors, the doctor may give additional doses of Konakion MM Paediatric.
How to give your baby Konakion MM Paediatric by mouth
If your baby was given Konakion MM Paediatric by mouth at birth, you will be asked to give your baby another 2 mg dose. You will give them this by mouth 4 to 7 days after birth.
If your baby is having breast milk and no formula milk you may be asked to give your baby 2 mg doses once a month (by mouth).
The pictures in this leaflet show you how to give the doses to your baby by mouth, using the dispenser provided in the pack. If you are not sure, or have any worries about doing this talk to your health visitor, midwife, doctor or pharmacist.
1. Ampoule and dispenser
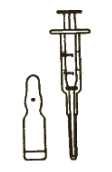
Picture 1
2. To open the ampoule
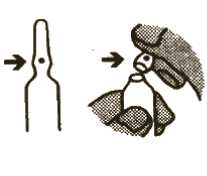
Picture 2
Please turn over
Konakion® MM Paediatric 2 mg/0.2 ml
solution for injection or oral administration Phytomenadione (vitamin K1)
Please refer to the Summary of Product Characteristics for full prescribing information.
Dosing information for preterm babies at birth for the prophylaxis of Vitamin K deficiency bleeding_
|
Weight of the baby |
Dose of vitamin K at birth |
Injection volume |
|
1 kg |
0.4 mg |
0.04 ml |
|
1.5 kg |
0.6 mg |
0.06 ml |
|
2 kg |
0.8 mg |
0.08 ml |
|
2.5 kg |
1 mg |
0.1 ml |
|
Over 2.5 kg |
1 mg |
0.1 ml |
10165127 GB FY
This information is intended for medical or healthcare professionals only:
The tear-off portion above is intended for the patient
INFORMATION FOR HEALTHCARE PROFESSIONALS
Presentation
Amber glass ampoules containing 0.2 ml solution. The solution is clear to slightly opalescent and pale yellow in colour. Excipients are glycocholic acid, lecithin, sodium hydroxide, hydrochloric acid and water for injections. Konakion MM Paediatric 2 mg/ 0.2 ml is essentially ‘sodium free’ as it contains less than 1 mmol sodium (2.64 mg per 1 ml). Cartons of 5 ampoules.
PACKS CONTAIN PLASTIC ORAL DISPENSERS. NOT TO BE USED FOR INJECTIONS.
Posology and method of administration Konakion MM Paediatric 2 mg/ 0.2 ml is for either injection (intravenous or intramuscular) or oral administration.
CAUTION: care is required when calculating and measuring the dose in relation to the baby’s weight (10 times dosing errors are common).
Prophylaxis of vitamin K deficiency bleeding (VKDB)
Healthy neonates of 36 weeks gestation and older:
Either:
• 1 mg administered by intramuscular injection at birth or soon after birth
or
• 2 mg orally at birth or soon after birth. The oral dose should be followed by a further dose of 2 mg at 4-7 days of age. A further 2 mg oral dose should be given at 1 month after birth. In exclusively formula fed infants the third oral dose can be omitted.
Preterm neonates of less than 36 weeks gestation weighing 2.5 kg or greater, and term neonates at special risk (e.g. prematurity, birth asphyxia, obstructive jaundice, inability to swallow, maternal use of anticoagulants or antiepileptics): 1 mg IM or IV at birth or soon after birth. The amount and frequency of further doses should be based on coagulation status.
Preterm neonates of less than 36 weeks gestation weighing less than 2.5 kg: 0.4 mg/kg (equivalent to 0.04 ml/kg) IM or IV at birth or soon after birth. This parenteral dose should not be exceeded. The amount and frequency of further doses should be based on coagulation status.
There is evidence that oral prophylaxis is insufficient in patients with underlying cholestatic liver disease and malabsorption.
CAUTION: care is required when calculating and measuring the dose in relation to the baby’s weight (10 times dosing errors are common).
Further oral doses in breast-fed infants have been advised, but safety or efficacy data for these additional doses is limited.
Therapy of early and/or late vitamin K deficiency bleeding (VKDB)
Initially 1 mg IV and further doses as required, depending on clinical picture and coagulation status. Konakion therapy may need to be accompanied by a more immediate effective treatment, such as transfusion of blood or blood clotting factors to compensate for severe blood loss and delayed response to vitamin K1.
Antidote therapy to anticoagulant drugs of the coumarin type
There have been no dose ranging studies performed to recommend a specific dose of Konakion MM Paediatric used as an antidote to anticoagulant drugs of the coumarin type in infants and children. Suggested doses are detailed below. Konakion MM Paediatric must be administered by intravenous injection in these patients. It is advisable that a haematologist is consulted about appropriate investigation and treatment in any infant or child in whom Konakion MM Paediatric is being considered.
For patients on warfarin therapy, therapeutic intervention must consider the reason for the patient being on warfarin and whether or not anticoagulant therapy has to be continued (e.g. in a patient with mechanical heart valve or repeated thrombo-embolic complications) as vitamin K administration is likely to interfere with anticoagulation with warfarin for 2 - 3 weeks.
For patients continuing to receive warfarin, the suggested dose for the partial reversal of anticoagulation is 30 micrograms/kg administered by IV injection. Konakion MM Paediatric is only suitable for the administration of doses of 30 micrograms/kg in children weighing over 13 kg.
The suggested dose of vitamin K for patients requiring a complete reversal of a warfarin overdose is 250-300 micrograms/kg administered by IV injection. It should be noted that the earliest effect seen with vitamin K treatment is at 4 to 6 hours and therefore, in patients with severe haemorrhage, replacement with coagulation factor concentrates may be indicated (discuss with haematologist). Konakion MM Paediatric is only suitable for the administration of doses of 250-300 micrograms/kg in children weighing over 1.6 kg. Prothrombin time should be measured 2 to 6 hours later and if the response has not been adequate, Konakion MM Paediatric administration may be repeated. Frequent monitoring of vitamin K dependent clotting factors is essential in these patients.
Method of administration
Konakion MM Paediatric can be administered by intramuscular or intravenous injection or by oral administration depending on the indication.
At the time of use, the ampoule contents should be clear. Following incorrect storage, the contents may become turbid or present a phase-separation. In this case the ampoule must not be used.
Parenteral use: For the administration of injection volumes of 0.04 ml (0.4 mg) to 0.1 ml (1 mg), 0.5 ml syringes with 0.01 ml gradations are recommended. Undiluted Konakion MM Paediatric is compatible with 0.5 ml syringes supplied by B.Braun.
Administration of Konakion MM Paediatric by i.v. infusion is not recommended because Konakion MM Paediatric must not be diluted or mixed with other parenteral medications. However, Konakion MM Paediatric may be administered by injecting the dose into the lower part of an infusion set containing 5% dextrose or 0.9% sodium chloride running at > 0.7 ml/minute, see section, Incompatibilities.
Oral use: For oral administration, oral dispensers are provided in the pack. After breaking the ampoule open, 0.2 ml of solution should be withdrawn into the oral dispenser until it reaches the mark on the dispenser (0.2 ml =
2 mg vitamin K). Drop the contents of the dispenser directly into the baby’s mouth by pressing the plunger.
Incompatibilities
Incompatibilities have been observed with diluted Konakion MM solution and certain siliconised syringes, therefore, Konakion MM Paediatric must not be diluted before injection.
Do not dilute with sodium chloride containing solutions as precipitation may occur.
Shelf life
Unopened: 3 years.
Special precautions for storage
Store below 25°C and protect from light. Do not freeze.
Do not use if the solution is turbid.
Date of preparation of leaflet May 2015
1 Please turn over ^
^ - Picture 1 shows the ampoule (the small glass ; container) and the dispenser. The part of the | dispenser which can be moved in and out is called the plunger.
| - Shake the ampoule until the liquid is in the ; bottom of the ampoule. Do not use it if it | looks cloudy.
; - Hold the bottom part of the ampoule between i the thumb and first finger of one hand. Make ; sure the spot is facing towards your thumb | (see Picture 2).
; - Hold the top of the ampoule between the i thumb and first finger of your other hand.
; Snap the top off by pushing away from the
side with the spot (see Picture 2).
5. How Konakion MM Paediatric is stored
• Konakion MM Paediatric ampoules should be stored in their original packaging to protect them from light.
• Konakion MM Paediatric should be stored at a temperature below 25°C.
• Keep out of the reach and sight of children.
• Do not use Konakion MM Paediatric after the expiry date printed on the pack.
• Do not throw away any whole left over ampoules. Instead, return them to your pharmacist so that they can be disposed of carefully. Only keep them if your doctor tells you to.
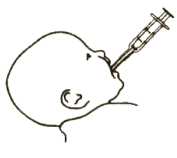
Picture 4
JL
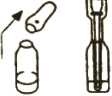
Picture 3
3. Put the dispenser into the ampoule. The tip of the dispenser should touch the bottom of the ampoule (see Picture 3). Pull the plunger up slowly to pull the medicine into the dispenser until it is level with the second mark (2 mg) on the side of the dispenser.
The dispenser is designed to draw up the right dose from the ampoule. There may be some liquid left over in the ampoule even after the right dose has been removed. This is OK.
Do not give your baby any extra liquid.
4. Put the dispenser into your baby’s mouth as shown in Picture 4. Gently push the plunger in, to give your baby the medicine.
If your baby gets more Konakion MM Paediatric than they should
If your baby has had more Konakion MM Paediatric than they should, talk to a doctor, nurse or midwife. The following effects may happen to your baby; jaundice (signs of which are yellowing of the skin or the whites of the eyes), tummy ache, constipation, soft stools (poo), seeming unwell, being agitated (upset), a rash and changes to how well their liver works (shown up by blood tests).
If you forget to give your baby Konakion MM Paediatric
• If you forget to give your baby their dose of Konakion MM Paediatric by mouth, talk to your health visitor, midwife or doctor about when to give the next dose.
• Do not give your baby a double dose to make up for a forgotten dose.
If someone else takes your baby’s Konakion MM Paediatric by mistake, they should talk to a doctor.
If you have any further questions on the use of this medicine, ask your doctor, nurse or midwife.
6. Further information
What Konakion MM Paediatric contains
The active substance in Konakion MM Paediatric 2 mg/0.2 ml is vitamin Ki (phytomenadione). Each 0.2 ml of liquid medicine contains 2 mg vitamin K1.
Other ingredients are glycocholic acid, sodium hydroxide, lecithin, hydrochloric acid and water for injections.
What Konakion MM Paediatric looks like and contents of the pack
Konakion MM Paediatric is a slightly opalescent, pale yellow liquid (‘solution for injection or oral administration’).
Konakion MM Paediatric is supplied in amber coloured glass ampoules in packs of 5 with plastic oral dispensers.
Marketing Authorisation Holder and Manufacturer
Roche Products Limited 6 Falcon Way Shire Park
Welwyn Garden City, AL7 1TW United Kingdom
This leaflet was last revised in May 2015
4. Possible side effects
Like all medicines, Konakion MM Paediatric can cause side effects, although not everyone gets them.
The following side effects may happen with this ; medicine:
Allergic reactions
The signs may include:
! • Swelling of your baby or child’s throat, face, lips and mouth. This may make it difficult for them to breathe or swallow.
; • Sudden swelling of your baby or child’s hands, feet and ankles.
; If your baby or child has an allergic reaction,
! tell a doctor straight away.
j A reaction where the injection was given
Rarely this may be severe. Signs include redness, swelling, pain and it may cause a scar.
; Reporting of side effects
I If you get any side effects, talk to your doctor or ; pharmacist or nurse. This includes any possible ! side effects not listed in this leaflet. You can also report side effects directly (see details below).
! By reporting side effects you can help provide ; more information on the safety of this medicine.
; United Kingdom
Yellow Card Scheme ; Website: www.mhra.gov.uk/yellowcard
2 ! 2 10165127 GB FY
10165127_315x420.indd 2 05.05.2015 15:03:48