Lactasol Solution For Haemofiltration And Haemodialysis
& -------------------------------X
Package leaflet: Information for the user
LACTASOL®
Solution for haemofiltration and haemodialysis
Sodium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate, sodium lactate solution 60% w/w
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• If any of the side effects become serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. WHAT LACTASOL IS AND WHAT IT IS USED FOR
2. BEFORE YOU ARE GIVEN LACTASOL
3. HOW TO USE LACTASOL
4. POSSIBLE SIDE EFFECTS
5. HOW TO STORE LACTASOL
6. FURTHER INFORMATION
1. WHAT LACTASOL IS AND WHAT IT IS USED FOR
Lactasol is used in hospitals in intensive care treatments called Continuous Renal Replacement Therapy. It is administered to correct any fluid and chemical imbalance of the blood, which is a consequence of kidney (renal) failure.
The treatments, using continuous renal replacement therapy, are designed to remove accumulated waste products from the blood when the kidneys are not functioning as they should.
The Lactasol solution is particularly used to treat critically ill patients with acute renal failure having a high concentration of potassium in the blood (hyperkalaemia).
Lactasol may also be used in cases of drug poisoning with dialysable or filterable substances.
2. BEFORE YOU ARE GIVEN LACTASOL
DO NOT USE LACTASOL DURING ANY OF THE FOLLOWING THREE CONDITIONS:
• if you have a low concentration of potassium in the blood (hy-pokalaemia)
• if you suffer from a very severe metabolic problem with too low pH in your blood (acidosis)
• if you have difficulties in metabolising lactate, an acidic component which should be converted into bicarbonate and used to increase the pH of your blood.
TAKE SPECIAL CARE WITH LACTASOL
Before and during treatment, your blood condition will be checked. For example, your acid- base balance and concentrations of salts (electrolytes) will be monitored.
Special attention should be given to the level of potassium in your blood to ensure the most appropriate potassium concentration.
You must tell your doctor if you are suffering from:
• liver disease
• heart disease
• general infection of your blood (sepsis)
USING OTHER MEDICINES
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. This is because the concentration in your blood of some of these medicines may be reduced during treatment with Lactasol.
Your doctor will decide if other medicines should be changed.
If you are treated with digitalis due to heart problems, the correction of the electrolyte concentration in your blood may lead to symptoms of digitalis overload. This is especially the case if your blood level of digitalis is higher than normally intended.
(UK)
PREGNANCY AND BREASTFEEDING
There is no adequate data from the use of Lactasol in pregnant or lactating women. Your doctor will decide whether you should be given Lactasol if you are pregnant or breast-feeding.
3. HOW TO USE LACTASOL
Lactasol is a sterile solution to be used in hospitals and administered by medical professionals only.
The volume (which corresponds to the dose) of Lactasol will depend on your condition. The dose volume will be determined by the physician, who is responsible for your treatment.
Lactasol can be administered into the venous blood line to replace fluid volume and electrolytes lost during the process of continuous haemofiltration or haemodiafiltration (specific methods of Continuous Renal Replacement Therapies). It can also be administered during continuous haemodialysis, where the sterile solution flows on one side of a dialysis membrane while the blood flows on the other side.
IF YOU ARE GIVEN MORE LACTASOL THAN YOU SHOULD BE GIVEN:
Your fluid balance and blood chemistry will be carefully monitored. Therefore, it is unlikely that you will be given more Lactasol than you should be given.
In the unlikely event that an overdose occurs, your doctor will take the necessary corrective measures and adjust your dose.
Overdose may result in electrolyte or acid-based disturbances as well as fluid overload if you suffer from renal failure. Overdose could also lead to problems with the heart.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Lactasol D05679001 Rev. 2012-12 3707 3
4. POSSIBLE SIDE EFFECTS
Like all medicines, Lactasol can cause side effects, although not everybody gets them.
The following side effects related to the use of Lactasol are possible:
• changes in levels of salt in your blood (electrolyte disturbances)
• low concentration of potassium in your blood (hypokalaemia), as Lactasol is potassium-free.
There are also some side effects which can be caused by the process of haemodialysis and haemofiltration, such as:
• feeling sick (nausea)
• being sick (vomiting)
• muscle cramps
• fits (convulsions)
• low blood pressure (hypotension)
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE LACTASOL
Keep out of reach and sight of children.
Do not use Lactasol after the expiry date which is stated on the label and the packaging.
The expiry date refers to the last day of that month.
Do not store below +4°C.
Do not use Lactasol if the solution is cloudy or if the over-wrap is damaged. All seals must be intact.
Lactasol can be disposed of via wastewater without harming the environment.
6. FURTHER INFORMATION WHAT LACTASOL CONTAINS
The active substances are: 1000 ml of solution contains:
Calcium chloride
Magnesium chloride (hexahydrate) 0.152 g
Sodium lactate solution 60% w/w 7.471 g
(corresponding to sodium lactate anhydrous 4.483 g)
Lactasol solution contains in mmol per litre:
Theoretical osmolarity:
287.5 mOsm/l
The other ingredients in Lactasol are:
• hydrochloric acid 3.646% w/v (for pH adjustment)
• water for injections
WHAT LACTASOL LOOKS LIKE AND CONTENTS OF THE PACK
Lactasol is presented in a one-compartment bag. The solution is clear and colourless.
Each bag contains 5000 ml solution for haemofiltration and haemodialysis. The bag is over-wrapped with a transparent film.
Each box contains two bags and one package leaflet.
MARKETING AUTHORISATION HOLDER
Gambro Lundia AB Box 10101 SE-220 10 Lund SWEDEN
MANUFACTURER
Gambro Dasco S.p.A.
Sondalo Plant Via Stelvio 94 23035 Sondalo (SO)
ITALY
This leaflet was last approved in 03/2010
4 Lactasol D05679001 Rev. 2012-12 3707
& -------------------------------X
The following information is intended for medical or healthcare professionals only
LACTASOL®
Solution for haemofiltration and haemodialysis
The solution is to be used only by or under the direction of a physician who should have sound experience of intensive care nursing and/or applied Continuous Renal Replacement Therapies such as haemofiltration, haemodiafiltration and haemodialysis.
The patient's haemodynamic, fluid, electrolyte and acid-base balance should be closely monitored throughout the procedure. Close monitoring of the potassium levels must be carried out to enable selection of the most appropriate potassium concentration.
Severe metabolic acidosis should be corrected with a bicarbonate solution prior to using a lactate based substitution fluid.
When used with a monitor, only monitors for Continuous Renal Replacement Therapy must be used.
The volume of Lactasol to be administered will depend on the patient's clinical condition and the target fluid balance. Continued application of haemofiltration, haemodiafiltration or haemodialysis will remove excess fluid and electrolytes.
Do not mix with bicarbonate containing solutions, as this may cause precipitation of calcium and magnesium carbonate.
Lactasol, when used as a substitution solution, is administered into the extracorporeal blood circuit before (pre-dilution) or after the he-mofilter (post-dilution).
In continuous haemodialysis or haemofiltration, the clearances obtained are directly proportional to the dialysate flow.
The range of flow rates used for the substitution solution in haemofiltration and haemodiafiltration are:
Adult: 500 - 1500 ml/hour
Children: 15 - 20 ml/kg/hour
The range of flow rates for the dialysis solution in haemodiafiltration or continuous haemodialysis are:
Adult: 500 - 2000 ml/hour
Children: 15 - 20 ml/kg/hour
INSTRUCTIONS FOR USE/ HANDLING
Aseptic technique shall be used throughout the administration to the patient.
Use only if the solution is clear and the over-wrap is undamaged. All seals must be intact. If leakage is discovered, discard the solution immediately since sterility can no longer be assured.
If heating of the solution to body temperature (37°C) is necessary the procedure must be carefully controlled verifying that the solution is clear and without particles.
The bag is fitted with an injection port for the possible addition of other necessary drugs. Medication shall only be added to the solution
(UK) under the responsibility of a physician in the following way:
Remove any fluid from the injection port, hold the bag upside down, insert the drug through the injection port and mix thoroughly. The solution must be administered immediately.
The dialysis or replacement fluid line may be connected to either of the two access ports.
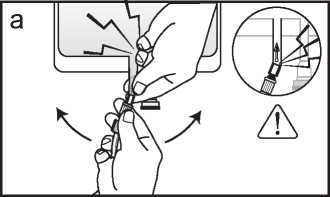
a. If the luer access is used, remove the cap and connect the male luer lock on the dialysis or replacement fluid line to the female luer connector on the bag; tighten. Using both hands, break the frangible pin at its base, and move it back and forth. Do not use a tool. Verify that the pin is completely separated and that the fluid is flowing freely. The pin will remain in the luer port during the treatment. (See figure a below)
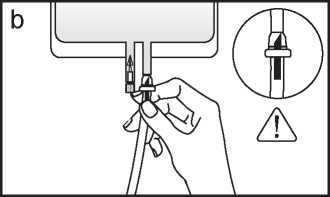
b. If the injection port is used, first remove the snap-off cap. Then introduce the spike through the rubber septum. Verify that the fluid is flowing freely. (See figure b below)
The solution is for immediate and single use only. Discard any unused solution immediately after use.
Lactasol D05679001 Rev. 2012-12 3707 11
Lactasol PL PVC UK AT DE PT EL 2012-12.indd 11 12/7/2012 11:36:49