Latanoprost Bausch & Lomb 0.05 Mg/ Ml Eye Drops Solution
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Latanoprost Bausch & Lomb, 50 micrograms/ml, eye drops, solution
Latanoprost
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Latanoprost Bausch & Lomb is and what it is used for
2. Before you use Latanoprost Bausch & Lomb
3. How to use Latanoprost Bausch & Lomb
4. Possible side effects
5. How to store Latanoprost Bausch & Lomb
6. Further information
1. WHAT LATANOPROST BAUSCH & LOMB IS AND WHAT IT IS USED FOR
The name of your medicine is Latanoprost Bausch & Lomb.
It is only for use in your eyes.
The active ingredient in Latanoprost Bausch & Lomb is one of a group of medicines known as prostaglandins. It lowers the pressure within your eye by increasing the natural flow of fluid from inside the eye out into the blood stream.
Latanoprost Bausch & Lomb is used to treat a type of glaucoma called open angle glaucoma and also a condition known as ocular hypertension. Both of these conditions can be linked with an increase in the pressure within your eye and eventually they may affect your eyesight.
2. BEFORE YOU USE LATANOPROST BAUSCH & LOMB DO NOT use Latanoprost Bausch & Lomb
- if you are allergic (hypersensitive) to the active substance latanoprost or any of the other ingredients of Latanoprost Bausch & Lomb (These are listed at section 6 of the leaflet.)
Take special care with Latanoprost Bausch & Lomb
Before using your medicine you should tell your doctor if:
- You have severe asthma, or your asthma is not well controlled.
- you have any other types of glaucoma.
- you have inflammatory conditions of your eye, eg conjunctivitis.
- you have no lens or an artificial lens in the eye (aphakia or pseudophakia).
- you have a closed or blocked retinal vein (retinal vein occlusion).
- you have diabetes which is affecting the eyes (diabetic retinopathy).
- you are susceptible to inflammation of the iris or the middle layer of the eye (iritis/uveitis).
- you are about to have or have had eye surgery.
Latanoprost Bausch & Lomb may cause a gradual change in the eye colour and this may become permanent. Ask your doctor for more information.
While using Latanoprost Bausch & Lomb you should have regular eye examinations.
Children
Latanoprost Bausch & Lomb is not recommended for the use in children/adolescents below 18 years due to a lack of data of safety and efficacy.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
You should not use other eyedrops which belongs to the same group of medicines (prostaglandins). Ask your doctor for advice if you are not sure.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
You should not use the eye drops if you are pregnant, think you might be pregnant or you are planning a pregnancy.
You should not use the eye drops if you are breast feeding a baby.
Driving and using machines
As with other eye drops, if your vision is blurred when you first put your drops in, wait until this wears off before you drive or operate machinery.
Important information about some of the ingredients of Latanoprost Bausch & Lomb
This medicinal product contains benzalkonium chloride which may cause eye irritation. Avoid contact with soft contact lenses. Remove contact lenses prior to application and wait at least 15 minutes before putting them back in. Benzalkonium chloride is known to discolour soft contact lenses.
Contact lens wearers:
Avoid contact with soft contact lenses. If you wear contact lenses, remove them prior to application and wait at least 15 minutes before reinsertion. The preservative benzalkonium chloride is known to discolour soft contact lenses.
3. HOW TO USE LATANOPROST BAUSCH & LOMB
Always use Latanoprost Bausch & Lomb exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Instructions for Latanoprost Bausch & Lomb use:
The usual dose is one drop of Latanoprost Bausch & Lomb into the affected eye(s) once daily.
The best time to do this is in the evening.
If you have to use other eye drops you should wait for at least 5 minutes before using them.
If you wear contact lenses, remove them before using Latanoprost Bausch & Lomb. You may reinsert them after 15 minutes.
This is the usual dose for adults, including elderly patients. Latanoprost Bausch & Lomb is not normally used in children.
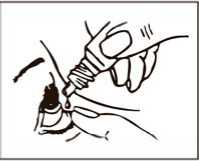
Follow the steps below to help you use Latanoprost Bausch & Lomb properly:
1. Wash your hands and sit or stand comfortably.
2. Twist off the cap.
3. Use your finger to gently pull down the lower eyelid of your affected eye.
4. Place the tip of the bottle close to, but not touching your eye.
5. Squeeze the bottle gently so that only one drop goes into your eye, then release the lower eyelid.
6. Press a finger against the corner of the affected eye by the nose. Hold for 1 minute whilst keeping the eye closed.
7. Repeat in your other eye if your doctor has told you to do this.
8. Put the cap back on the bottle.
How long should you use your medicine for?
Latanoprost Bausch & Lomb should be used until your doctor tells you to stop.
If you use more Latanoprost Bausch & Lomb than you should
Be careful when you are squeezing the bottle so that you only put one drop into the affected eye. If you put too many drops in your eye, you may feel some slight irritation in the eye. Ask your doctor for advice.
If Latanoprost Bausch & Lomb is accidentally swallowed, you should contact your doctor.
If you forget to use Latanoprost Bausch & Lomb
If you forget to use your eye drops at the usual time, wait until it is time for your next dose.
Do not put an extra drop into your eye to make up for the one you may have missed.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Latanoprost Bausch & Lomb can cause side effects, although not everybody gets them.
Stop using Latanoprost Bausch & Lomb and contact your doctor immediately if:.
- you notice changes in your vision that did not always occur just after administering the drops. These changes may mean that reading and being able to see fine detail is difficult.
- you feel as if there is something in your eye(s).
- your eye(s) water and become red or painful and/or your vision becomes blurred.
- your eye lid(s) look swollen, puffy or feel sore.
The evaluation of the side effects is based on the following frequencies:
• Very common side effects (affects more than 1 user in 10)
• Common side effects (affects 1 to 10 users in 100)
• Uncommon side effects (affects 1 to 10 users in 1,000)
• Rare side effects (affects 1 to 10 users in 10,000)
• Very rare: affects less than 1 user in 10,000
• Not known: frequency cannot be estimated from the available data.
Eye Disorders:
Very common:
- Over an extended period of treatment there may be a gradual and slight
change in eye colour. This is typically seen in the first 8 months. Patient's most likely to be affected are those with mixed coloured irises, e.g. blue-brown, grey-brown, yellow-brown and green-brown. The colour change is slight in most of cases and not observed clinically. The Iris may appear more brownish, due to an increase in melanin pigmentation. Ask your doctor for more information (increased iris pigmentation);
- red eye due to increased blood supply (mild to moderate conjunctival
hyperaemia);
- eye irritation (burning grittiness, itching, stinging and foreign body
sensation);
- eyelash and vellus hair changes (increased length, thickness, pigmentation
and number)
Common:
- temporary punctate epithelial erosions of the cornea, mostly without
symptoms;
- Inflammation of the eyelid (blepharitis);
- eye pain.
Uncommon:
- swelling of the eyelid due to excess fluid retention (eyelid oedema);
- dry eye;
- Inflammation of the cornea (keratitis);
- vision blurred;
- conjunctivitis.
Rare:
- Inflammation of the iris or middle layer of the eye (iritis/uveitis) (the
majority of reports in patients with concomitant risk factors);
- swelling of the area at the back of the eye responsible for seeing fine detail
(macular oedema);
- swelling or erosions of the corneal (symptomatic corneal oedema and
erosions); blurred vision or the appearance of halos around lights
- swelling around the eyes (periorbital oedema);
- eyelashes may grow in the "wrong" direction sometimes resulting in eye
irritation;
- extra row of lashes (distichiasis).
Cardiac Disorders:
Very rare:
- patients with angina have experienced worsening of their chest pain
symptoms.
Frequency not known:
- feeling your heartbeat (palpitations).
Respiratory Disorders:
Rare:
- asthma,
- worsening of asthma and breathlessness.
Skin Disorders:
Uncommon:
- skin rash.
Rare:
- localised skin reaction on the eyelids;
- darkening of the eyelids and the skin around the eyes.
General Disorders
Very rare:
- chest pain.
Frequency not known:
- headache,
- dizziness.
Musculoskeletal and Connective Tissue Disorders:
Frequency not known:
- pain in the muscles (myalgia);
- pain in the joints (arthralgia).
If you experience any of these side effects and are concerned please tell your doctor as soon as possible.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE LATANOPROST BAUSCH & LOMB
Before Latanoprost Bausch & Lomb is first opened, Store and transport refrigerated (2°C - 8°C).
Keep the bottle in the outer carton in order to protect from light.
After opening, do not store above 25°C.
Each bottle should be thrown away 4 weeks after first opening.
Do not use Latanoprost Bausch & Lomb after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Keep out of the reach and sight of children.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Latanoprost Bausch & Lomb contains
The active substance is latanoprost. Each ml contains 50 micrograms latanoprost.
2.5 ml of eye drops, solution (content of a bottle) contains 125 micrograms of latanoprost.
The other ingredients are:
- benzalkonium chloride 0.2 mg/ml
- sodium chloride
- sodium dihydrogen phosphate monohydrate
- disodium phosphate anhydrous
- water, purifed
What Latanoprost Bausch & Lomb looks like and contents of the pack
The eye drops are a colourless or pale-yellow, clear solution.
Each bottle contains 2.5 ml eye drops, solution.
Latanoprost Bausch & Lomb is available in the following pack sizes: one bottle of
2.5 ml, 3 bottles of 2.5 ml and 6 bottles of 2.5 ml.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Dr. Gerhard Mann Chem.-pharm. Fabrik GmbH Brunsbutteler Damm 165-173 13581 Berlin Germany
Telephone:+49 (0)30 33093-0 Fax:+49 (0)30 33093-350
This medicinal product is authorized in the Member States of the EEA under the following names:
|
Austria, Bulgaria, Czech Republic, Estonia, Greece, Italy, Latvia, Lithuania, Luxembourg, The Netherlands, Poland, Romania, Slovak Republic, Spain |
Arulatan |
|
Belgium |
Latanoprost Dr. Mann Pharma |
|
France, Portugal |
Latanoprost Dr. Gerhard Mann Chem.-pharm. Fabrik |
|
Germany |
Latan-Ophtal |
|
Hungary |
Lanotan |
|
United Kingdom |
Latanoprost Bausch & Lomb |