Latanoprost/Timolol 50 Micrograms/Ml + 5 Mg/Ml Eye Drops Solution
Out of date information, search anotherPackage Leaflet: Information for the user
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others.
It may harm them, even if their signs of illness are the same as yours.
• It you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
• Latanoprost / Timolol 50 micrograms/ ml + 5mg/ ml Eye Drops, Solution is referred to as 'these eye drops' in this leaflet.
If you use these eye drops more than you should
If you put too many drops in your eye you may experience some minor irritation in your eye and they may water and turn red. This should pass but if you are worried contact your doctor for advice.
If you swallow these eye drops If you accidentally swallow these eye drops you should contact your doctor for advice. If you swallow a lot of these eye drops you may feel sick, have stomach pains, feel tired, flushed and dizzy and start to sweat.
If you forget to use these eye drops
Carry on with
your next dose at the usual time.
Do not use a double dose to make for the dose you have forgotten.
If you are unsure about anything talk to your doctor or pharmacist.
If you stop using these eye drops If you want to stop using this medicine talk to your doctor first. If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
What is in this leaflet:
1. What these eye drops are and what they are used for
2. What you need to know before you use these eye drops
3. How to use these eye drops
4. Possible side effects
5. How to store these eye drops
6. Contents of the pack and other information
1. WHAT THESE EYE DROPS ARE AND WHAT THEY ARE USED FOR
2. WHAT YOU NEED TO KNOW BEFORE YOU USE THESE EYE DROPS
5. HOW TO STORE THESE EYE DROPS
These eye drops contain two active ingredients: latanoprost and timolol. Latanoprost belongs to a group of medicines known as prostaglandin analogues. It increases the flow of fluid out of the eye.
Timolol belongs to a group of medicines known as beta-blockers. It slows down the production of the fluid in the eye. These eye drops are used to treat
• Open angle glaucoma or
• Ocular hypertension
These are both caused by too much pressure in the eye.
Do not use these eye drops:
• if you are allergic to Latanoprost or Timolol, beta- blockers or any of the other ingredients (listed in section 6)
• if you have now or have had in the past respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• if you have serious heart problems or heart rhythm disorders
If you are unsure if you can use these eye drops please tell your doctor.
Tell your doctor before you have an operation that you are using these eye drops as timolol may change the effects of some medicines used during anaesthesia.
Warnings and precaution
Talk to your doctor or pharmacist before using these eye drops if you have now or have had in the past
• coronary heart disease - symptoms can include chest pain or tightness, breathlessness or choking, heart failure, low blood pressure (hypotension)
• disturbances of heart rate such as slow heart beat
• breathing problems, asthma or chronic obstructive pulmonary disease
• poor blood circulation disease such as Raynaud's disease or Raynaud's syndrome
• diabetes, as timolol may mask signs and symptoms of low blood sugar
• any kind of eye surgery (including cataract surgery) as timolol may change effects of some medicines used during anaesthesia.
• eye problems (such as eye pain, eye irritation, eye inflammation or blurred vision)
• dry eyes
• you wear contact lenses. You can still use these eye drops but follow the instructions for contact lens wearers in Section 3
• you know that you suffer from angina (particularly a type known as Prinzmetal angina)
• overactivity of the thyroid gland as timolol may mask signs and symptoms
• think you have developed a thyroid problem while using these eye drops
• severe allergic reactions and need to carry adrenaline/epinephrine
• a viral infection of the eye caused by the herpes simplex virus (HSV)
These eye drops are used for open angle glaucoma and ocular hypertension. Tell your doctor if you have any other type of glaucoma.
These eye drops work by reducing the pressure in your eye by:
• Slowing down the production of fluid
• Increasing the amount of fluid that is drained from your eye
If these conditions are not treated they may affect your eyesight. Your doctor will usually prescribe these eye drops for you when other medicines have not worked.
These eye drops can cause permanent colour change in your eye.
They can also affect your eyelashes and the skin around eye (see section 4)
Children and adolescents
There is limited experience with these eye drops in children and adolescents.
Other medicines and these eye drops
These eye drops can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines,
• Medicines to lower blood pressure
• Heart medicines
• Medicines to treat diabetes
• Any medicine containing prostaglandins
• Adrenaline (also known as epinephrine)
• Medicines to treat high blood pressure (hypertension)
• Quinidine (used to treat heart conditions and some types of malaria), antidepressants known as fluoxetine and paroxetine.
Pregnancy and breast-feeding Do not use these eye drops if you are pregnant or trying to get pregnant unless your doctor considers it necessary. Tell your doctor immediately if you are pregnant, think you are pregnant or are planning to become pregnant.
Do not use these eye drops if you are breast-feeding. Timolol may get into your milk. Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
When you use these eye drops your vision may become blurred for a short time. If this happens to you, do not drive or use any tools or machines until your vision becomes clear again.
If you wear contact lenses
• Do not use these eye drops while your contact lenses are in your eyes
• Remove your contact lenses before using these eye drops
• Wait at least 15 minutes after using these eye drops before putting your contact lenses back in.
These eye drops contains benzalkonium chloride These eye drops contains a preservative called benzalkonium chloride. This preservative may cause eye irritation and can discolour soft contact lenses.
Like all medicines, these eye drops can cause side effects, although not everybody gets them.
Most side effects are related to the eye: These eye drops may cause a gradual change in your eye colour and this may become permanent.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using these eye drops without speaking to your doctor.
It is also possible that these eye drops might cause serious changes in the way your heart works. If you notice changes in your heart rate or are feeling tired and breathless you should speak to a doctor and tell them you have been using these eye drops.
The following are side effects of these eye drops.
The frequency of possible side effects listed below is defined using the following convention:
Very common (may affect more than 1 in 10 people)
Common (may affect up to 1 in 10 people )
Uncommon (may affect up to 1 in 100 people)
Rare (may affect up to 1 in 1000 people to 10 users in 10,000)
Very rare (may affect up to 1 in 10,000 people)
Not known (frequency cannot be estimated from the available data)
Very common:
• A gradual change in your eye colour (an increase in the amount of brown pigment in the coloured part of your eye). If you have mixed-colour eyes (blue-brown, grey-brown, yellow brown or green-brown) you are more likely to see this change than if you have eyes of one colour (blue, grey, green or brown eyes). Any changes in your eye colour may take years to develop. The colour change may be permanent and may be more noticeable if you use these eye drops in only one eye. There appears to be no problems associated with the change in eye colour. Your eye colour will not continue to change after you have stopped taking these eye drops.
Common:
• Eye irritation (a feeling of burning, grittiness, itching, stinging or the sensation of a foreign body in the eye) and eye pain.
Uncommon:
• Headache.
• Redness of the eye, eye infection (conjunctivitis), blurred vision, watery eyes, swelling (inflammation) of the eyelids, irritation or damage to the surface of the eye
• Skin rashes or itching Other side effects
Although not seen with these eye drops, the following additional side effects have been seen with Latanoprost and Timolol, which are in these eye drops. They might occur when you use these eye drops.
Timolol, like other medicines applied into the eyes, is absorbed into the blood. This may cause similar side effects as seen with intravenous and/ or oral as applicable beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example, taken by mouth or injected.
Listed side effects include reactions seen with Latanoprost and Timolol when
Keep this medicine out of the sight and reach of children.
Store the unopened bottle of eye drops in a refrigerator (between 2°C and 8°C). After opening the bottle it is not necessary to store it in a refrigerator but do not store it above 25°C.
When you are not using these eye drops, keep the bottle in the outer carton, in order to protect from light.
After opening do not use the eye drops in this bottle for more than 4 weeks.
used for treating eye conditions:
• Generalised allergic reactions including swelling beneath the skin that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, hives or itchy rash, localised and generalised rash, itchiness, severe sudden life-threatening allergic reaction.
• Developing a viral infection of the eye caused by the herpes simplex virus (HSV)
• Symptoms of allergic reaction (swelling and redness of the skin and rash)
• Low blood glucose levels
• Depression, memory loss, decreased sex drive, difficulty sleeping (insomnia), nightmares
• Dizziness, unusual sensations (like pins and needles), stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), sudden fainting or feeling you may faint, and headache
• Changes to the eyelashes and fine hairs around the eye (increased number, length, direction of growth, thickness and darkening), signs and symptoms of eye irritation (e.g. burning, stinging, itching, tearing, redness), inflammation of eyelid, inflammation in the cornea, blurred vision and detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the front layer of the eyeball), drooping of the upper eyelid (making the eye stay half closed),double vision.
• Problems with your vision, sensitivity to light, fluid filled cyst within the coloured part of the eye (iris cyst)
• Ringing in the ears (tinnitus)
• Problems with your heart such as slow heart rate, chest pains, awareness of heart beat (palpitations), oedema (fluid build up), worsening of angina, changes in rhythm or speed of the heartbeat and congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure
• Low blood pressure, Raynaud's phenomenon and cold hands and feet
• Asthma, worsening of asthma, shortness of breath, difficulty breathing , cough, constriction of the airways in the lungs (predominantly in patients with pre- existing disease)
• Feeling sick (nausea), diarrhoea, heartburn, dry mouth, taste disturbances, nausea, indigestion, abdominal pain, vomiting
• Darkening of the eyelids, hair loss/baldness, skin rash, skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis
• Joint pain, muscle pain not caused by exercise
• Chest pain, muscle weakness, tiredness and swelling
• Sexual dysfunction, decreased libido. If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Do not use these eye drops after the expiry date which is stated on the carton and bottle label after EXR The expiry date refers to the last day of that month. Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
3. HOW TO USE THESE EYE DROPS
Always use these eye drops exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose for adults is one drop once a day in each affected eye. These eye drops are not recommended if you are aged under 18.
After using these eye drops, press a finger into the comer of your eye, by the nose (Picture 2) for 2 minutes. This helps to stop timolol getting into the rest of the body.
Use these eye drops as instructed by your doctor until your doctor tells you to stop. Your doctor may want you to have extra checks on your heart and circulation if you use these eye drops.
Instructions for use:
• First wash your hands
• Remove your contact lenses
• If you are using the bottle for the first time, snap off the protective cover by turning it clockwise to break the seal.
• Unscrew the cap
• Tilt your head back and look at the ceiling
• Pull the lower eyelid gently downwards
d
• Hold the bottle upside down above the eye and gently squeeze the bottle to release a drop into your eye
• Avoid touching the eye (or any other surface) with the tip of the bottle
• Close the affected eye. Press your fingertip against the inside comer of the closed eye by the nose, and hold for 2 minutes. This is important because it reduces the amount of latanoprost and timolol getting into the rest of your body.
• Repeat for the other eye if necessary
• Put the cap back on the bottle and tighten onto the nozzle after every use.
If you use this medicines with other
eye drops
Wait at least 5 minutes between using these
eye drops and using other eye drops.
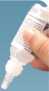
Picture 1


Picture 2
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What these eye drops contain
The active substances are latanoprost and timolol
Each ml contains 50 micrograms latanoprost and 5 mg timolol (as 6.8 mg timolol maleate)
The other ingredients (excipients) are: Sodium chloride Benzalkonium chloride Sodium dihydrogen phosphate monohydrate
Disodium phosphate anhydrous Hydrochloric acid solution and Sodium hydroxide solution (for pH adjustment)
Water for injections
What these eye drops look like and
contents of the pack
Each carton contains one bottle of these
eye drops. Each bottle contains 2.5 ml of
these eye drops.
These eye drops are clear and colourless liquid.
These eye drops are available in pack sizes of 1,3 and 6. Not all pack sizes may be marketed
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
FDC Pharma
Unit 6, Fulcrum 1, Solent Way, Whiteley, Fareham, Hampshire,
P015 7FE, UK Tel: + 44 (0) 1489 565222 Fax: + 44 (0) 1489 565222 Email: fdcil@btconnect.com
Manufacturer:
FDC International Ltd.,
Unit 6, Fulcrum 1, Solent Way, Whiteley, Fareham, Hampshire,
P015 7FE, UK PL number: PL 35638/0004 This leaflet was last revised in April 2012.