Lecicarbon A Suppository
PACKAGE LEAFLET: INFORMATION FOR THE USER
LericarborfA Suppository
Active substances:
sodium hydrogen carbonate, sodium dihydrogen phosphate.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Always use this medicine exactly as described in this leaflet or as your doctor or pharmacist have told you.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must talk to a doctor if you do not feel better or if you feel worse after 3 days.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Wh.il is in I his le.illel:
1. What Lecicarbon® A Suppository is and what it is used for
2. What you need to know before you use Lecicarbon® A Suppository
3. How to use Lecicarbon® A Suppository
4. Possible side effects
5. How to store Lecicarbon® A Suppository
6. Contents of the pack and other information
1. WHAT LECICARBON® A SUPPOSITORY IS AND WHAT IT IS USED FOR
Lecicarbon® works to relieve the symptoms of frequent or chronic constipation by slowly releasing carbon dioxide after insertion in the rectum. The carbon dioxide is produced in excess and triggers an evacuation of the bowels within 15 to 30 minutes.
Lecicarbon® can be used in patients:
- with constipation caused by low-fibre food or insufficient exercise (e.g. bedridden patients)
- to evacuate the bowels before surgery, diagnostic procedures or childbirth
- to ease bowel movements after an operation
- in addition to other laxatives which have proven to be ineffective
2. WHAT YOU NEED TO KNOW BEFORE YOU USE LECICARBON® A SUPPOSITORY
Do not use Lecicarbon® A Suppository
- if you are allergic (hypersensitive) to sodium hydrogen carbonate, sodium dihydrogen phosphate or any of the other ingredients of Lecicarbon® A Suppository.
- in cases of intestinal obstruction (ileus).
Warnings and |'ie.nili"iis
If you suffer from an enlargement of the end of the large intestine due to various causes (toxic megacolon) the product should only be used with explicit permission of the doctor.
Other medicines and Lecicarbon® A Suppository
You can use Lecicarbon® A Suppository while taking other medicines.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Using Lecicarbon® A Suppository with food and drink
There are no special precautions required.
Pregnancy and breast-feeding Pregnancy:
You may use Lecicarbon® A Suppository during pregnancy after consulting your doctor.
Breast feeding:
You may use Lecicarbon® A Suppository during breast feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Lecicarbon® A Suppository has no influence on the abilities to drive or to use machines, although the onset of action (defecation) is expected after 15 to 30 minutes.
Important information about some of the ingredients of Lecicarbon® A Suppository
This medicinal product contains (3-sn-Phosphatidyl)choline (=soya lecithin, phosphatides).
If you are allergic to peanut or soya, do not use this medicinal product.
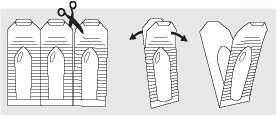
3. HOW TO USE LECICARBON® A SUPPOSITORY
Unless otherwise directed, insert one suppository into the rectum when needed. If necessary, this can be repeated after 30 to 60 minutes. Before Lecicarbon® A Suppository can be used, remove from the packaging by separating one suppository from the strip at the perforation.
Grasp the plastic tabs in both hands and pull apart. Insert the suppository through the anus.
Insertion is made easier if the suppository is first dipped into water. For a child, it may be necessary to press the two halves of the child's bottom together to avoid the suppository being immediately expelled.
The onset of action is after 15 to 30 minutes. Do not use oil or petroleum jelly as a lubricant.
Lecicarbon® A Suppository is suitable for adults and children over 12 years of age.
Lecicarbon® C Suppository is suitable for children under 12 years of age.
Lecicarbon® A Suppository is safe to use for long periods and are not habit-forming. However, a doctor should always be consulted in cases of chronic constipation.
If you use more Lecicarbon® A Suppository than you should
The usual dose is one suppository. If necessary, the administration can be repeated after 30-60 minutes. There is no danger if you exceed this dose, but it is not sensible to administer more than two suppositories.
If you forget to use Lecicarbon® A Suppository
Lecicarbon® A Suppository is only used when needed.
If you stop using Lecicarbon® A Suppository
Lecicarbon® A Suppository is only used when needed.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Lecicarbon® A Suppository can cause side effects, although not everybody gets them.
Lecicarbon® A Suppository does not normally cause problems (side effects). In rare cases Lecicarbon® can cause a slight burning sensation after insertion which quickly disappears.
You should tell your doctor or pharmacist if you experience any side effects not listed in this leaflet.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at www.mhra.gov.uk/yellowcard. Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy.
5. HOW TO STORE LECICARBON® A SUPPOSITORY
Keep this medicine out of the sight and reach of children.
Do not store above 25' C.
Do not use Lecicarbon® A Suppository after the expiry date which is stated on the carton and on the blister shell after "EXP". The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Lecicarbon® A Suppository contains
Each suppository of Lecicarbon® A Suppository contains Sodium hydrogen carbonate 0.500 g, sodium dihydrogen phosphate 0.680 g.
- The active substances are sodium hydrogen carbonate and sodium dihydrogen phosphate.
- The other ingredients are (3-sn-Phosphatidyl)choline (=soya lecithin, phosphatides), hard fat and silica, colloidal anhydrous.
What Lecicarbon® A Suppository looks like and contents of the pack
Lecicarbon® A Suppository is a cream coloured, homogenous, torpedo shaped suppository.
Available pack sizes: 10, 30 or 100 suppositories Hospital pack sizes: 50 x 10 or 500 suppositories.
Not all pack sizes are marketed.
Marketing Authorisation Holder and Manufacturer
Milk ling \||I||"IIV|I|..|| I M4 i
athenstaedt GmbH a Co KG BahnhofstraBe 11 82515 Wolfratshausen Germany
M.iiiuI.p tur i
Haupt Pharma Livron S.A.S.
1 Rue Comte de Sinard 26250 livron-Sur-Drome France
Tel.: +33 (4) 75 61 02 26 Fax: +33 (4) 75 61 02 36
I his 2 alle was last r vised in "2/2"! 2