Levetiracetam Amneal 100 Mg/Ml Oral Solution
60 mm
M
Pharmacode position can change as per Supplier’s m/c requirement & additional small pharma code may appear on the front/back panel
■fk
F PH;
|
Weight |
Starting dose: 0.1 ml/kg twice daily |
Maximum dose: 0.3 ml/kg twice daily |
|
6 kg |
0.6 ml twice daily |
1.8 ml twice daily |
|
8 kg |
0.8 ml twice daily |
2.4 ml twice daily |
|
10 kg |
1 ml twice daily |
3 ml twice daily |
|
15 kg |
1.5 ml twice daily |
4.5 ml twice daily |
|
20 kg |
2 ml twice daily |
6 ml twice daily |
|
25 kg |
2.5 ml twice daily |
7.5 ml twice daily |
|
From 50 kg |
5 ml twice daily |
15 ml twice daily |
|
Weight |
Starting dose: 0.07 ml/kg twice daily |
Maximum dose: 0.21 ml/ kg twice daily |
|
4 kg |
0.3 ml twice daily |
0.85 ml twice daily |
|
5 kg |
0.35 ml twice daily |
1.05 ml twice daily |
|
6 kg |
0.45 ml twice daily |
1.25 ml twice daily |
|
7 kg |
0.5 ml twice daily |
1.5 ml twice daily |
amneal'
PHARMACEUTICALS
Package Leaflet: Information for the patient Levetiracetam Amneal 100 mg/ml oral solution
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Levetiracetam Amneal is and what it is used for
2. What you need to know before you take Levetiracetam Amneal
3. How to take Levetiracetam Amneal
4. Possible side effects
5. How to store Levetiracetam Amneal
6. Contents of the pack and other information
1. What Levetiracetam Amneal is and what it is used for
Levetiracetam Amneal 100 mg/ml oral solution is an antiepileptic medicine (a medicine used to treat seizures in epilepsy).
Levetiracetam Amneal is used:
• on its own in adults and adolescents from 16 years of age with newly diagnosed epilepsy, to treat partial onset seizures with or without secondary generalisation
• as an add-on to other antiepileptic medicines to treat:
• partial onset seizures with or without generalisation in adults, adolescents, children and infants from one month of age
• myoclonic seizures in adults and adolescents from 12 years of age with juvenile
myoclonic epilepsy,
• primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with idiopathic generalised epilepsy
2. What you need to know before you take Levetiracetam Amneal Do not take Levetiracetam Amneal
• If you are allergic to levetiracetam or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
Talk to your doctor or pharmacist before taking Levetiracetam Amneal
• If you suffer from kidney problems, follow your doctor’s instructions. He/ she may decide if your dose should be adjusted.
• If you notice any slow down in the growth or unexpected puberty development of your child, please contact your doctor.
• If you notice an increase in seizure severity (e.g. increased number), please contact your doctor.
• A small number of people being treated with anti-epileptics such as Levetiracetam have had thoughts of harming or killing themselves. If you have any symptoms of depression and/or suicidal ideation, please contact your doctor.
Other medicines and Levetiracetam Amneal
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Levetiracetam Amneal with food, drink and alcohol
You may take Levetiracetam Amneal with or without food. As a safety precaution, do not take Levetiracetam Amneal with alcohol.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking this medicine.
If you are pregnant or if you think you may be pregnant, please inform your doctor.
Levetiracetam Amneal should not be used during pregnancy unless clearly necessary. A risk of birth defects for your unborn child cannot be completely excluded. Levetiracetam Amneal has shown unwanted reproductive effects in animal studies at dose levels higher than you would need to control your seizures.
Breast-feeding is not recommended during treatment.
Driving and using machines
Levetiracetam Amneal may impair your ability to drive or operate any tools or machinery, as Levetiracetam Amneal may make you feel sleepy. This is more likely at the beginning of treatment or after an increase in the dose. You should not drive or use machines until it is established that your ability to perform such activities is not affected.
Levetiracetam contains methyl parahydroxybenzoate, propyl parahydroxybenzoate and maltitol
Levetiracetam oral solution includes methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions (possibly delayed).
doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to take Levetiracetam Amneal
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Levetiracetam Amneal must be taken twice a day, once in the morning and once in the evening, at about the same time each day.
Take the oral solution following your doctor’s instructions.
Monotherapy
Dose in adults and adolescents (from 16 years of age):
General dose: between 10 ml (1000 mg) and 30 ml (3000 mg) each day, divided in 2 intakes per day. When you will first start taking Levetiracetam Amneal, your doctor will prescribe you a lower dose during 2 weeks before giving you the lowest general dose.
Add-on therapy
Dose in adults and adolescents (12 to 17 years) weighing 50 kg or more:
General dose: between 10 ml (1000 mg) and 30 ml (3000 mg) each day, divided in 2 intakes per day.
Dose in infants (6 to 23 months), children (2 to 11 years) and adolescents (12 to 17 years) weighing less than 50 kg:
Your doctor will prescribe the most appropriate pharmaceutical form of Levetiracetam Amneal according to the age, weight and dose.
General dose: between 0.2 ml (20 mg) and 0.6 ml (60 mg) per kg bodyweight each day, divided in 2 intakes per day. The exact quantity of oral solution formulation should be delivered using the syringe provided in the cardboard box.
Dose in infants (1 month to less than 6 months):
General dose: between 0.14 ml (14 mg) and 0.42 ml (42 mg) per kg bodyweight each day, divided in 2 intakes per day. The exact quantity of oral solution formulation should be delivered using the syringe provided in the cardboard box.
Method of administration:
Levetiracetam Amneal oral solution may be diluted in a glass of water or baby’s bottle.
Instructions for use:
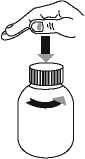
• Open the bottle: press the cap and turn it anticlockwise (figure 1)
©
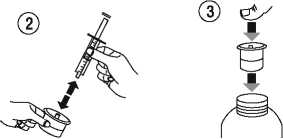
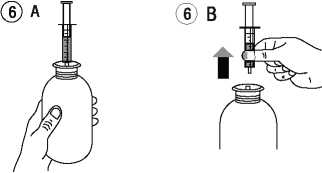
Separate the adaptor from the syringe (figure 2). Insert the adaptor into the bottle neck (figure 3). Ensure it is well fixed.
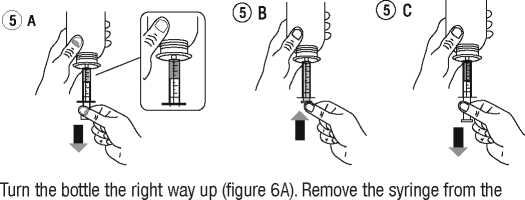
• Take the syringe and put it in the adaptor opening (figure 4). Turn the bottle upside down (figure 5).
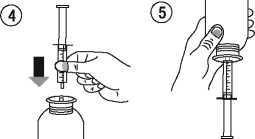
• Fill the syringe with a small amount of solution by pulling the piston down (figure 5 A), then push the piston upward in order to remove any possible bubble (figure 5 B). Pull the piston down to the graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (figure 5 C).
Size: 210 x 420 mm Font type: Helvetica Neue LT Com
Font size: 9 pt.
Levetiracetam oral solution also contains maltitol. If you have been told by your
N
Outer line not to be printed-
jf
h—h
10 mm
M
5 mm
Levetiracetam - Leaflet - UK
Review -2
n
adaptor (figure 6B).
,mf
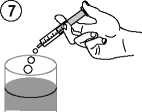
• Empty the contents of the syringe in a glass of water or baby's bottle by pushing the piston to the bottom of the syringe (figure 7).
• Drink the whole contents of the glass/baby's bottle.
• Close the bottle with the plastic screw cap.
• Wash the syringe with water only (figure 8)
Duration of treatment:
• Levetiracetam Amneal is used as a chronic treatment. You should continue Levetiracetam Amneal treatment for as long as your doctor has told you.
• Do not stop your treatment without your doctor’s advice as this could increase your seizures. Should your doctor decide to stop your Levetiracetam Amneal treatment, he/she will instruct you about the gradual withdrawal of Levetiracetam Amneal.
If you take more Levetiracetam Amneal than you should:
The possible side effects of an overdose of Levetiracetam Amneal are sleepiness, agitation, aggression, decrease of alertness, inhibition of breathing and coma.
Contact your doctor if you took more Levetiracetam Amneal than you should. Your doctor will establish the best possible treatment of overdose.
If you forget to take Levetiracetam Amneal:
Contact your doctor if you have missed one or more doses. Do not take a double dose to make up for a forgotten dose.
If you stop taking Levetiracetam Amneal:
If stopping treatment, as with other antiepileptic medicines, Levetiracetam Amneal should be discontinued gradually to avoid an increase of seizures.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some of the side effects like sleepiness, tiredness and dizziness may be more common at the beginning of the treatment or at dose increase. These effects should however decrease over time.
Very common: may affect more than 1 in 10 people
• nasopharyngitis
• somnolence (sleepiness), headache.
Common: may affect up to 1 in 10 people
• anorexia (loss of appetite);
• depression, hostility or aggression, anxiety, insomnia, nervousness or irritability;
• convulsion, balance disorder (equilibrium disorder), dizziness (sensation of unsteadiness), lethargy, tremor (involuntary trembling);
• vertigo (sensation of rotation);
• cough;
• abdominal pain, diarrhoea, dyspepsia (indigestion), vomiting, nausea;
• rash;
• asthenia/fatigue (tiredness).
Uncommon: may affect up to 1 in 100 people
• decreased number of blood platelets, decreased number of white blood cells;
• weight decrease, weight increase;
• suicide attempt and suicidal ideation, mental disorder, abnormal behaviour, hallucination, anger, confusion, panic attack, emotional instability/mood swings, agitation;
• amnesia (loss of memory), memory impairment (forgetfulness), abnormal coordination/ataxia (impaired coordinated movements), paraesthesia (tingling), disturbance in attention (loss of concentration);
• diplopia (double vision), vision blurred;
• liver function test abnormal;
• hair loss, eczema, pruritus;
• muscle weakness, myalgia (muscle pain);
• injury.
Rare: may affect up to 1 in 1,000 people
• infection;
• decreased number of all blood cell types;
• severe hypersensitivity reactions (DRESS);
• decreased blood sodium concentration;
• suicide, personality disorders (behavioural problems), thinking abnormal (slow thinking, unable to concentrate);
• uncontrollable muscle spasms affecting the head, torso and limbs, difficulty in controlling movements, hyperkinesia (hyperactivity);
• pancreatitis;
• hepatic failure, hepatitis;
• skin rash, which may form blisters and looks like small targets (central dark spots surrounded by a paler area, with a dark ring around the edge) (erythema multiforme), a widespread rash with blisters and peeling skin, particularly around the mouth, nose, eyes and genitals (Stevens-Johnson syndrome), and a more severe form causing skin peeling in more than 30% of the body surface (toxic epidermal necrolysis).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www. mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Levetiracetam Amneal
Keep this medicine out of the sight and reach of children.
This medicinal product does not require any special storage conditions.
Do not use after 7 months of first opening the bottle.
Do not use this medicine after the expiry date stated on the carton after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Levetiracetam Amneal contains
The active substance is called levetiracetam. Each ml contains 100 mg of levetiracetam.
The other ingredients are: maltitol liquid (E965), glycerol (E422), propylene glycol, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), citric acid monohydrate, sodium citrate (dihydrate), acesulfame potassium (E950), mafco magnasweet (Glycerine Monoammonium glycyrrhizinate), grape flavour (flavourings, propylene glycol, ascorbic acid), purified water.
What Levetiracetam Amneal looks like and contents of the pack
Levetiracetam Amneal 100 mg/ml oral solution is a clear, colourless, grape flavoured liquid.
300 ml amber glass bottle (type III) with a white child resistant closure in a cardboard box also containing a 10 ml oral syringe (graduated every 0.25 ml) and an adaptor for the syringe.
150 ml amber glass bottle (type III) with a white child resistant closure in a cardboard box also containing a 3 ml oral syringe (graduated every 0.1 ml) and an adaptor for the syringe.
150 ml amber glass bottle (type III) with a white child resistant closure in a cardboard box also containing a 1 ml oral syringe (graduated every 0.05 ml) and an adaptor for the syringe.
Marketing Authorisation Holder
Amneal Pharma Europe Limited 70 Sir John Rogerson’s Quay Dublin 2 Ireland
Manufacturer
APL Swift Services (Malta) Limited HF26, Hal Far Industrial Estate,
Hal Far, Birzebbugia, BBG 3000 Malta
This leaflet was last revised in 07/2014
Size: 210 X 420 mm Font type: Helvetica Neue LT Com
Font size: 9 pt.
M
Outer line not to be printed-
h—H
10 mm
M
5 mm