Levetiracetam Mylan 100 Mg/Ml Oral Solution
Package Leaflet: Information for the patient
Package Leaflet: Information for the patient
|
Weight |
Starting dose: 0.1 ml/kg twice daily |
Maximum dose: 0.3 ml/kg twice daily |
|
6 kg |
0.6 ml twice daily |
1.8 ml twice daily |
|
8 kg |
0.8 ml twice daily |
2.4 ml twice daily |
|
10 kg |
1 ml twice daily |
3 ml twice daily |
|
15 kg |
1.5 ml twice daily |
4.5 ml twice daily |
|
20 kg |
2 ml twice daily |
6 ml twice daily |
|
25 kg |
2.5 ml twice daily |
7.5 ml twice daily |
|
From 50 kg |
5 ml twice daily |
15 ml twice daily |
|
Weight |
Starting dose: 0.07 ml/kg twice daily |
Maximum dose: 0.21 ml/kg twice daily |
|
4 kg |
0.3 ml twice daily |
0.85 ml twice daily |
|
5 kg |
0.35 ml twice daily |
1.05 ml twice daily |
|
6 kg |
0.45 ml twice daily |
1.25 ml twice daily |
|
7 kg |
0.5 ml twice daily |
1.5 ml twice daily |
Take the syringe and pull back the plunger a little way (Figure 2).
Push the tip of the syringe into the adaptor opening. Push the plunger down slowly to introduce air inside the bottle (Figure 3).
Levetiracetam Mylan 100 mg/ml oral solution
(levetiracetam)
Read all of this leaflet carefully before you or your child start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Levetiracetam Mylan is and what it is used for
2. What you need to know before you take Levetiracetam Mylan
3. How to take Levetiracetam Mylan
4. Possible side effects
5. How to store Levetiracetam Mylan
6. Contents of the pack and other information
1. What Levetiracetam Mylan is and what it is used for
Levetiracetam 100 mg/ml oral solution contains levetiracetam, which is an antiepileptic medicine (a medicine used to treat seizures in epilepsy). Levetiracetam is used:
• on its own in adults and adolescents from 16 years of age with newly diagnosed epilepsy, to treat a certain form of epilepsy. Epilepsy is a condition where the patients have repeated fits (seizures). Levetiracetam is used for the epilepsy form in which the fits initially affect only one side of the brain, but could thereafter extend to larger areas on both sides of the brain (partial onset seizure with or without secondary generalisation). Levetiracetam has been given to you by your doctor to reduce the number of fits.
• as an add-on to other antiepileptic medicines to treat:
* partial onset seizures with or without generalisation in adults, adolescents, children and infants from one month of age
* myoclonic seizures (short, shock-like jerks of a muscle or group of muscles) in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy
* primary generalised tonic-clonic seizures (major fits, including loss of consciousness) in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy (the type of epilepsy that is thought to have a genetic cause)
2. What you need to know before you take Levetiracetam Mylan
Do not take Levetiracetam:
• If you are allergic to levetiracetam or any of the other ingredients of this medicine (listed in section 6).
• If you are allergic to medicines known as 'pyrrolidone derivatives' such as piracetam or ethosuximide (used in the treatment of epilepsy) or povidone (an ingredient in some medicines).
Warnings and precautions
Talk to your doctor or pharmacist before taking
Levetiracetam:
• If you suffer from kidney problems, follow your doctor's instructions. He/she may decide that your dose should be adjusted.
• If you have severe liver problems as your doctor may need to carry out some blood tests and he/she may decide that your dose should be adjusted.
During treatment:
• a small number of people being treated with antiepileptics such as levetiracetam have had thoughts of harming or killing themselves. If you have any symptoms of depression and/or thoughts of harming or killing yourself, please contact your doctor.
Children and adolescents During treatment:
• if you notice any slow down in the growth or unexpected puberty development of your child, please contact your doctor.
Levetiracetam must not be used on its own (monotherapy) by children and adolescents below 16 years.
Other medicines and Levetiracetam
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription. Levetiracetam may not work as effectively if you take it with some macrogol containing oral laxatives (medicines used to treat constipation). If you need to take these medicines together you should take oral laxatives containing macrogol one hour before or one hour after taking this medicine.
Levetiracetam with alcohol
As a safety precaution, do not take levetiracetam
with alcohol.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Levetiracetam should not be used during pregnancy unless clearly necessary. It is not known if levetiracetam will harm your unborn baby. Levetiracetam has shown unwanted reproductive side effects in animals at dose levels higher than you would take to control your seizures.
If you are a woman of child-bearing age and not using contraception, talk to your doctor before taking this medicine.
Breast-feeding
Levetiracetam can pass into breast milk and could cause side effects in your baby. Therefore breast-feeding is not recommended during treatment.
Driving and using machines Levetiracetam may impair your ability to drive or operate any tools or machinery, as it may make you feel sleepy or dizzy. This is more likely to happen at the beginning of your treatment or after an increase in the dose. You should not drive or use machines until it is established that your ability to perform such activities is not affected.
Levetiracetam contains methyl parahydroxybenzoate, propyl parahydroxybenzoate and maltitol
Levetiracetam includes methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions (possibly delayed). Levetiracetam also contains maltitol liquid. If you have been told by your doctor that you have an intolerance to some sugars, talk to your doctor before taking this medicine.
3. How to take Levetiracetam Mylan
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Levetiracetam must be taken by mouth (orally) twice a day, once in the morning and once in the evening, at about the same time each day. Take the oral solution following your doctor's instructions.
You may take Levetiracetam with or without food. Monotherapy
Dose in adults and adolescents (from 16 years of age):
The recommended dose is between 10 ml (1,000 mg) and 30 ml (3,000 mg) each day, divided into two intakes per day.
When you first start taking levetiracetam, your doctor will prescribe you a lower dose (500 mg each day) for 2 weeks before giving you the lowest recommended dose of 1000 mg.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box. Add-on therapy
Dose in adults and adolescents (12 to 17 years) weighing 50 kg or more:
The recommended dose is between 10 ml (1,000 mg) and 30 ml (3,000 mg) each day, divided into two intakes per day.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box. Dose in infants (6 to 23 months), children (2 to 11 years) and adolescents (12 to 17 years) weighing less than 50 kg:
Your doctor will prescribe the most appropriate pharmaceutical form of levetiracetam according to the age, weight and dose.
The oral solution is more appropriate for infants and children under the age of 6 years.
The recommended dose is between 0.2 ml (20 mg) and 0.6 ml (60 mg) per kilogram bodyweight each day, divided into two intakes per day.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box.
Dose in infants (1 month to less than 6 months):
The recommended dose is between 0.14 ml (14 mg) and 0.42 ml (42 mg) per kilogram bodyweight each day, divided into two intakes per day.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box.
Method of administration:
Levetiracetam may be diluted in a glass of water or baby's bottle and can be taken with or without food. Instruction for use:
Instruction for use for 1 ml and 3 ml syringes with an adaptor
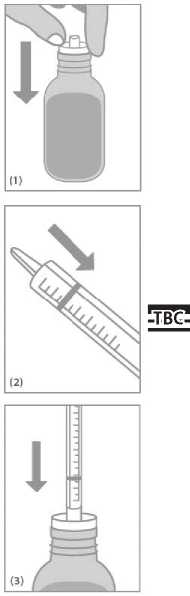
Open the bottle and push the syringe adaptor firmly into the bottle neck (Figure 1).
Turn the bottle upside down with the syringe still in place (Figure 4).
Pull the plunger down and fill the syringe with a quantity of solution slightly beyond the prescribed dose (Figure 5).
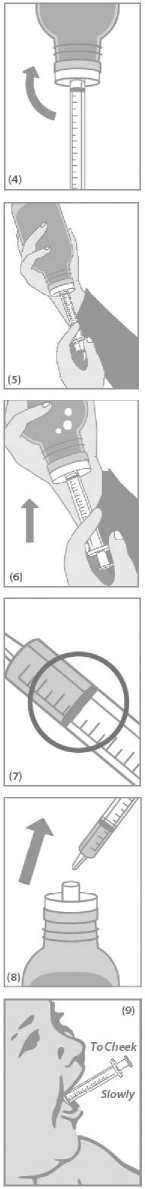
Push the plunger in slowly to the graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (Figure 7).
Turn the bottle the right way up and remove the syringe (Figure 8).
If any bubbles appear in the syringe keep the bottle upside down and slightly push in the plunger and pull it back again. Repeat until there are no bubbles in the syringe (Figure 6).
For young children gently put the tip of the syringe into the child's mouth to the inside of the cheek. Push the plunger in slowly and allow the child to swallow the content of the syringe (Figure 9). The content of the syringe can also be emptied in a glass of water or baby's bottle. Be sure that your child drinks the whole contents of the glass or bottle.
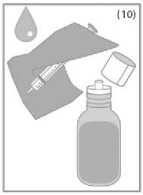
Wash the syringe with water after use and close the bottle with the plastic screw cap (Figure 10).
If you take more Levetiracetam than you should
The possible side effects of an overdose of levetiracetam are sleepiness, agitation, aggression, decreased alertness, inhibition of breathing and coma.
Contact your doctor if you took more Levetiracetam than you should. Your doctor will establish the best possible treatment of overdose.
If you forget to take Levetiracetam Contact your doctor if you have missed one or more doses.
Do not take a double dose to make up for a forgotten dose.
If you stop taking Levetiracetam
Levetiracetam is used as a chronic treatment. You should continue Levetiracetam treatment for as long as your doctor has told you. Do not stop your treatment without your doctor's advice as this could increase your seizures. Should your doctor decide to stop your Levetiracetam, he/she will instruct you about the gradual withdrawal of levetiracetam.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects,
although not everybody gets them.
Tell your doctor immediately, stop taking Levetiracetam and go to your nearest hospital casualty department straight away if you have any of the following serious side effects; you may need medical attention:
Common (may affect up to 1 in 10 people):
• fits (convulsion)
Uncommon (may affect up to 1 in 100 people):
• attempting to harm or kill yourself (suicide attempt), thoughts of harming or killing yourself (suicidal ideation), mental disorder, changes in behaviour (abnormalbehaviour), seeing, feeling or hearing things that are not there (hallucination)
• decreased number of white blood cells (leukopenia) -you may notice an increase in infections such as sore throat, mouth ulcers etc. with fever.
Rare (may affect up to 1 in 1,000 people):
• suicide
• skin rash, which may form blisters and looks like small targets (central dark spots surrounded by a paler area, with a dark ring around the edge) (erythema multiforme), a widespread rash with blisters and peeling skin, particularly around the mouth, nose, eyes and genitals (Stevens-Johnson syndrome), and a more severe form causing skin peeling in more than 30 % of the body surface (toxic epidermal necrolysis).
• severe allergic reactions causing swelling of the face, lips, tongue or throat (anaphylaxis, angioedema, DRESS).
• inflammation of the pancreas (pancreatitis). You may feel sick and have upper abdominal swelling and pain that radiates into the back.
• liver failure or inflammation of the liver(hepatic failure/ hepatitis). You may feel sick, notice yellowing of the skin and eyes, and have abdominal pain and swelling.
• decreased number of all blood cell types (pancytopenia) - you may notice an increase in infections such as sore throat, mouth ulcers etc. with fever, an increase in unexpected bruising or bleeding, or feel tired, breathless and weak.
Other possible side effects Some of the side effects like sleepiness, tiredness and dizziness may be more common at the beginning of the treatment or at dose increase. These effects should however decrease over time.
Very common (may affect more than 1 in 10 people):
• inflammation and congestion of the nasal passages and of the upper part of the throat (nasopharyngitis)
• sleepiness (somnolence), headache.
Common (may affect up to 1 in 10 people):
• loss of appetite (anorexia)
• feeling depressed, hostile or aggressive, feeling anxious (anxiety), difficulty falling and staying asleep at night (insomnia), feeling nervous or irritable
• balance disorder (equilibrium disorder), a sensation of unsteadiness (dizziness), lethargy, involuntary trembling (tremor)
• a sensation of rotation and loss of balance (vertigo)
• cough
• abdominal pain, diarrhoea, indigestion (dyspepsia), being sick (vomiting), feeling sick (nausea)
• rash
• weakness and lack of energy (asthenia/fatigue). Uncommon (may affect up to 1 in100 people):
• decreased number of blood platelets, which may be seen in blood tests (thrombocytopenia).
• weight decrease, weight increase
• anger, confusion, panic attack, emotional instability/ mood swings, agitation
• loss of memory (amnesia), forgetfulness (memory impairment), impaired coordinated movements (abnormal coordination/ataxia), tingling (paraesthesia), disturbance in attention (loss of concentration)
• double vision (diplopia), vision blurred
• liver function test abnormal
• hair loss, which may be reversible (alopecia), eczema, itchy skin (pruritus)
• muscle weakness, muscle pain (myalgia)
• injury.
Rare (may affect up to 1 in 1,000 people):
• infection
• decreased blood sodium concentration
(hyponatraemia)
• personality disorders (behavioural problems), thinking abnormal (slow thinking, unable to concentrate)
• uncontrollable muscle spasms affecting the head, torso and limbs (choreoathetosis), difficulty in controlling movements (dyskinesia), hyperactivity (hyperkinesia).
Reporting of side effects If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Levetiracetam Mylan
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the cardboard box and bottle after EXP. The expiry date refers to the last day of the month.
Store in the original container in order to protect from light.
Do not use after 4 months of first opening the bottle.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Levetiracetam contains
The active substance is called levetiracetam. Each ml contains 100 mg of levetiracetam.
The other ingredients are: sodium citrate, citric acid monohydrate (both used for pH adjustment), methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), ammonium glycyrrhizate, glycerol (E422), maltitol liquid (E965), acesulfame potassium (E950), grape flavour (contains propylene glycol), purified water.
What Levetiracetam looks like and contents of the pack
Levetiracetam 100 mg/ml oral solution is a clear, faint coloured solution.
The 300 ml glass bottle of Levetiracetam (for infants and young children aged from 6 months to less than 4 years) is packed in a cardboard box containing a 3 ml oral syringe (graduated every 0.1 ml) and an adaptor for the syringe.
The 300 ml glass bottle of Levetiracetam (for infants aged 1 month to less than 6 months) is packed in a cardboard box containing a 1 ml oral syringe (graduated every 0.05 ml) and an adaptor for the syringe. Marketing Authorisation Holder Mylan,
Potters Bar, Hertfordshire,
EN6 1TL, United Kingdom Manufacturer Balkanpharma-Troyan AD 1 Krayrechna Str,
Troyan 5600 Bulgaria
This leaflet was last revised in July 2016
942221
|
Weight |
Starting dose: 0.1 ml/kg twice daily |
Maximum dose: 0.3 ml/kg twice daily |
|
6 kg |
0.6 ml twice daily |
1.8 ml twice daily |
|
8 kg |
0.8 ml twice daily |
2.4 ml twice daily |
|
10 kg |
1 ml twice daily |
3 ml twice daily |
|
15 kg |
1.5 ml twice daily |
4.5 ml twice daily |
|
20 kg |
2 ml twice daily |
6 ml twice daily |
|
25 kg |
2.5 ml twice daily |
7.5 ml twice daily |
|
From 50 kg |
5 ml twice daily |
15 ml twice daily |
|
Weight |
Starting dose: 0.07 ml/kg twice daily |
Maximum dose: 0.21 ml/kg twice daily |
|
4 kg |
0.3 ml twice daily |
0.85 ml twice daily |
|
5 kg |
0.35 ml twice daily |
1.05 ml twice daily |
|
6 kg |
0.45 ml twice daily |
1.25 ml twice daily |
|
7 kg |
0.5 ml twice daily |
1.5 ml twice daily |
Levetiracetam Mylan 100 mg/ml oral solution
(levetiracetam)
Read all of this leaflet carefully before you or
your child start taking this medicine because it
contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Levetiracetam Mylan is and what it is used for
2. What you need to know before you take Levetiracetam Mylan
3. How to take Levetiracetam Mylan
4. Possible side effects
5. How to store Levetiracetam Mylan
6. Contents of the pack and other information
1. What Levetiracetam Mylan is and what it is used for
Levetiracetam 100 mg/ml oral solution contains levetiracetam, which is an antiepileptic medicine (a medicine used to treat seizures in epilepsy).
Levetiracetam is used:
• on its own in adults and adolescents from 16 years of age with newly diagnosed epilepsy, to treat a certain form of epilepsy. Epilepsy is a condition where the patients have repeated fits (seizures). Levetiracetam is used for the epilepsy form in which the fits initially affect only one side of the brain, but could thereafter extend to larger areas on both sides of the brain (partial onset seizure with or without secondary generalisation). Levetiracetam has been given to you by your doctor to reduce the number of fits.
• as an add-on to other antiepileptic medicines to treat:
* partial onset seizures with or without generalisation in adults, adolescents, children and infants from one month of age
* myoclonic seizures (short, shock-like jerks of a muscle or group of muscles) in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy
* primary generalised tonic-clonic seizures (major fits, including loss of consciousness) in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy (the type of epilepsy that is thought to have a genetic cause)
2. What you need to know before you take Levetiracetam Mylan
Do not take Levetiracetam:
• If you are allergic to levetiracetam or any of the other ingredients of this medicine (listed in section 6).
• If you are allergic to medicines known as 'pyrrolidone derivatives' such as piracetam or ethosuximide (used in the treatment of epilepsy) or povidone (an ingredient in some medicines).
Warnings and precautions
Talk to your doctor or pharmacist before taking Levetiracetam:
• If you suffer from kidney problems, follow your doctor's instructions. He/she may decide that your dose should be adjusted.
• If you have severe liver problems as your doctor may need to carry out some blood tests and he/she may decide that your dose should
be adjusted.
During treatment:
• a small number of people being treated with antiepileptics such as levetiracetam have had thoughts of harming or killing themselves. If you have any symptoms of depression and/or thoughts of harming or killing yourself, please contact your doctor.
Children and adolescents
During treatment:
• if you notice any slow down in the growth or unexpected puberty development of your child, please contact your doctor.
Levetiracetam must not be used on its own (monotherapy) by children and adolescents below 16 years.
Other medicines and Levetiracetam
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
Levetiracetam may not work as effectively if you take it with some macrogol containing oral laxatives (medicines used to treat constipation). If you need to take these medicines together you should take oral laxatives containing macrogol one hour before or one hour after taking this medicine.
Levetiracetam with alcohol
As a safety precaution, do not take levetiracetam with alcohol.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Levetiracetam should not be used during pregnancy unless clearly necessary. It is not known if levetiracetam will harm your unborn baby. Levetiracetam has shown unwanted reproductive side effects in animals at dose levels higher than you would take to control your seizures.
If you are a woman of child-bearing age and not using contraception, talk to your doctor before taking this medicine.
Breast-feeding
Levetiracetam can pass into breast milk and could cause side effects in your baby. Therefore breastfeeding is not recommended during treatment.
Driving and using machines
Levetiracetam may impair your ability to drive or operate any tools or machinery, as it may make you feel sleepy or dizzy. This is more likely to happen at the beginning of your treatment or after an increase in the dose. You should not drive or use machines until it is established that your ability to perform such activities is not affected.
Levetiracetam contains methyl parahydroxybenzoate, propyl parahydroxybenzoate and maltitol
Levetiracetam includes methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) which may cause allergic reactions (possibly delayed).
Levetiracetam also contains maltitol liquid. If you have been told by your doctor that you have an intolerance to some sugars, talk to your doctor before taking this medicine.
3. How to take Levetiracetam Mylan
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Levetiracetam must be taken by mouth (orally) twice a day, once in the morning and once in the evening, at about the same time each day. Take the oral solution following your doctor's instructions.
You may take Levetiracetam with or without food. Monotherapy
Dose in adults and adolescents (from 16 years of age):
The recommended dose is between 10 ml (1,000 mg) and 30 ml (3,000 mg) each day, divided into two intakes per day.
When you first start taking levetiracetam, your doctor will prescribe you a lower dose (500 mg each day) for 2 weeks before giving you the lowest recommended dose of 1000 mg.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box.
Add-on therapy
Dose in adults and adolescents (12 to 17 years) weighing 50 kg or more:
The recommended dose is between 10 ml (1,000 mg) and 30 ml (3,000 mg) each day, divided into two intakes per day.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box.
Dose in infants (6 to 23 months), children (2 to 11 years) and adolescents (12 to 17 years) weighing less than 50 kg:
Your doctor will prescribe the most appropriate pharmaceutical form of levetiracetam according to the age, weight and dose.
The oral solution is more appropriate for infants and children under the age of 6 years.
The recommended dose is between 0.2 ml (20 mg) and 0.6 ml (60 mg) per kilogram bodyweight each day, divided into two intakes per day.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box.
Dose in infants (1 month to less than 6 months):
The recommended dose is between 0.14 ml (14 mg) and 0.42 ml (42 mg) per kilogram bodyweight each day, divided into two intakes per day.
The exact quantity of oral solution should be measured using the oral syringe provided in the cardboard box.
Method of administration:
Levetiracetam may be diluted in a glass of water or baby's bottle and can be taken with or without food.
Instruction for use:
Instruction for use for 10 ml syringes
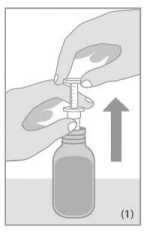
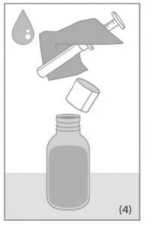
Open the bottle. Before starting the measuring procedure make sure that the transparent dosing body of the syringe as well as the white plunger are in the bottom most position.
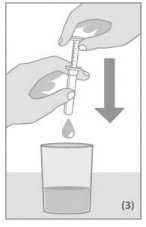
Place the syringe into the bottle. To measure the dosing quantity, use one hand to hold the dosing body and the other hand to pull up the plunger until you reach the graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (Figure 1).
Pull the syringe by the dosing body out of the bottle (Figure 2).

Empty the contents of the syringe into a glass of water by pushing down the plunger. Be sure to drink the whole contents of the glass. The contents of the syringe can also be given directly from the syringe into the mouth or emptied onto a spoon (Figure 3).
Wash the syringe with water after use and close the bottle with the plastic screw cap (Figure 4).
If you take more Levetiracetam than you should
The possible side effects of an overdose of levetiracetam are sleepiness, agitation, aggression, decreased alertness, inhibition of breathing and coma.
Contact your doctor if you took more Levetiracetam than you should. Your doctor will establish the best possible treatment of overdose.
If you forget to take Levetiracetam
Contact your doctor if you have missed one or more doses.
Do not take a double dose to make up for a forgotten dose.
If you stop taking Levetiracetam
Levetiracetam is used as a chronic treatment. You should continue Levetiracetam treatment for as long as your doctor has told you. Do not stop your treatment without your doctor's advice as this could increase your seizures. Should your doctor decide to stop your Levetiracetam, he/she will instruct you about the gradual withdrawal of levetiracetam.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately, stop taking Levetiracetam and go to your nearest hospital casualty department straight away if you have any of the following serious side effects; you may need medical attention:
Common (may affect up to 1 in 10 people):
• fits (convulsion)
Uncommon (may affect up to 1 in 100 people):
• attempting to harm or kill yourself (suicide attempt), thoughts of harming or killing yourself (suicidalideation), mental disorder, changes in behaviour (abnormalbehaviour), seeing, feeling or hearing things that are not there (hallucination)
• decreased number of white blood cells (leukopenia) - you may notice an increase in infections such as sore throat, mouth ulcers etc. with fever.
Rare (may affect up to 1 in 1,000 people):
• suicide
• skin rash, which may form blisters and looks like small targets (central dark spots surrounded by a paler area, with a dark ring around the edge) (erythema multiforme), a widespread rash with blisters and peeling skin, particularly around the mouth, nose, eyes and genitals (Stevens-Johnson syndrome), and a more severe form causing skin peeling in more than 30 % of the body surface (toxic epidermal necrolysis).
• severe allergic reactions causing swelling of the face, lips, tongue or throat (anaphylaxis, angioedema, DRESS).
• inflammation of the pancreas (pancreatitis). You may feel sick and have upper abdominal swelling and pain that radiates into the back.
• liver failure or inflammation of the liver(hepatic failure/hepatitis). You may feel sick, notice yellowing of the skin and eyes, and have abdominal pain and swelling.
• decreased number of all blood cell types (pancytopenia) - you may notice an increase in infections such as sore throat, mouth ulcers etc. with fever, an increase in unexpected bruising or bleeding, or feel tired, breathless and weak.
Other possible side effects
Some of the side effects like sleepiness, tiredness and dizziness may be more common at the beginning of the treatment or at dose increase. These effects should however decrease over time.
Very common (may affect more than 1 in 10 people):
• inflammation and congestion of the nasal passages and of the upper part of the throat
(nasopharyngitis)
• sleepiness (somnolence), headache.
Common (may affect up to 1 in 10 people):
• loss of appetite (anorexia)
• feeling depressed, hostile or aggressive, feeling anxious (anxiety), difficulty falling and staying asleep at night (insomnia), feeling nervous
or irritable
• balance disorder (equilibrium disorder), a sensation of unsteadiness (dizziness), lethargy, involuntary trembling (tremor)
• a sensation of rotation and loss of balance (vertigo)
• cough
• abdominal pain, diarrhoea, indigestion (dyspepsia), being sick (vomiting), feeling sick (nausea)
• rash
• weakness and lack of energy (asthenia/fatigue).
Uncommon (may affect up to 1 in100 people):
• decreased number of blood platelets, which may be seen in blood tests (thrombocytopenia).
• weight decrease, weight increase
• anger, confusion, panic attack, emotional instability/mood swings, agitation
• loss of memory (amnesia), forgetfulness (memory impairment), impaired coordinated movements (abnormal coordination/ataxia), tingling (paraesthesia), disturbance in attention (loss
of concentration)
• double vision (diplopia), vision blurred
• liver function test abnormal
• hair loss, which may be reversible (alopecia), eczema, itchy skin (pruritus)
• muscle weakness, muscle pain (myalgia)
• injury.
Rare (may affect up to 1 in 1,000 people):
• infection
• decreased blood sodium concentration
(hyponatraemia)
• personality disorders (behavioural problems), thinking abnormal (slow thinking, unable
to concentrate)
• uncontrollable muscle spasms affecting the head, torso and limbs (choreoathetosis), difficulty in controlling movements (dyskinesia), hyperactivity (hyperkinesia).
Reporting of side effects If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Levetiracetam Mylan
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the cardboard box and bottle after EXP. The expiry date refers to the last day of the month.
Store in the original container in order to protect from light.
Do not use after 4 months of first opening the bottle.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Levetiracetam contains
The active substance is called levetiracetam. Each ml contains 100 mg of levetiracetam.
The other ingredients are: sodium citrate, citric acid monohydrate (both used for pH adjustment), methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), ammonium glycyrrhizate, glycerol (E422), maltitol liquid (E965), acesulfame potassium (E950), grape flavour (contains propylene glycol), purified water.
What Levetiracetam looks like and contents of the pack
Levetiracetam 100 mg/ml oral solution is a clear, faint coloured solution.
The 300 ml glass bottle of Levetiracetam (for children aged 4 years and above, adolescents and adults) is packed in a cardboard box containing a 10 ml oral syringe (graduated every 0.25 ml).
Marketing Authorisation Holder
Mylan,
Potters Bar, Hertfordshire,
EN6 1TL, United Kingdom
Manufacturer
Balkanpharma-Troyan AD 1 Krayrechna Str,
Troyan 5600 Bulgaria
This leaflet was last revised in July 2016
942229