Levosert 20 Microgram/24 Hours Intrauterine Delivery System
1 /^\ /■✓-nq OTf-* 20 micrograms/24 hours
LC VUoCI L Intrauterine Delivery System
Levonorgestrel
Information for the user
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
1. What Levosert® is and what it is used for
2. What you need to know before you use Levosert®
3. How to use Levosert®
4. Possible side effects
5. How to store Levosert®
6. Contents of the pack and other information
1. What Levosert® is and what it is used for
Levosert® is an intrauterine delivery system (IUS) for insertion in the womb. It can be used in the following ways:
- As an effective method of contraception (prevention of pregnancy).
- For heavy menstrual bleeding (heavy periods).
Levosert® prevents pregnancy by controlling the monthly development of the womb lining so that it is not thick enough for you to become pregnant; by making the normal mucus in the opening to the womb (the cervical canal) thicker so that the sperm cannot get through to fertilise the egg; by preventing the release of eggs (ovulation) in some women.
There are also local effects on the lining of the womb caused by the presence of the T-shaped frame.
Levosert® is also useful for reducing menstrual blood flow, so it can be used if you suffer from heavy menstrual bleeding (periods). This is called menorrhagia. The hormone in Levosert® acts by controlling the monthly development of the womb lining, making it thinner, so that there is less bleeding every month.
Levosert® is placed inside the uterus (womb) where it slowly releases the hormone levonorgestrel over a period of 3 years or until it is removed.
2. What you need to know before you use Levosert®
Before you have Levosert® fitted, your doctor or nurse will carry out some tests to make sure that Levosert® is suitable for you to use. This will include a pelvic examination and may also include other examinations such as a breast examination, if your doctor or nurse feels this is appropriate.
Genital infections will need to be successfully treated before you can have Levosert® fitted.
If you have epilepsy, tell the doctor or nurse fitting Levosert® because, although rare, a fit can occur during insertion. Some women might feel faint after the procedure. This is normal and your doctor or nurse will tell you to rest for a while.
Not all women can use
Levosert®. Do not use Levosert®
and tell your doctor ifyou:
• are pregnant, suspect that you are pregnant or are breast feeding
• have an unusual vaginal bleeding pattern
• have an abnormal womb or fibroids
• have an unusual or unpleasant vaginal discharge, or vaginal itching
• have or have had pelvic inflammatory disease
• have or have had inflammation of the lining of your womb following delivery of your baby
• have or have had an infection of the womb after delivery or after abortion during the past 3 months
• have or have had inflammation of the cervix (neck of your womb)
• have or have had an abnormal smear test (changes in the cervix)
• have had a stroke, heart attack or any heart problems
• have or have had liver problems
• have any condition which makes you susceptible to infections. A doctor will have told you if you have this
• have or have had any type of cancer, suspected cancer or leukaemia (blood cancer)
• have or have had trophoblastic disease (the trophoblast provides nutrients to the foetus). A doctor will have told you ifyou have this
• are allergic (hypersensitive) to the levonorgestrel or any of the other ingredients this medicine (listed in section 6).
Talk to your doctor before using
Levosert® ifyou:
• are diabetic (too high blood glucose level), have high blood pressure or abnormal blood lipid levels
• have fits (epilepsy)
• have a history of blood clots (thrombosis)
• are on long term steroid therapy
• are taking any other medicines as some medicines may
stop Levosert® from working properly
• have or develop migraine, dizziness, blurred vision, unusually bad headaches or if you have headaches more often than before
• have ever had an ectopic pregnancy (development of the foetus outside the womb) or a history of ovarian cysts
• have yellowing of the skin or whites of the eyes
• cancer affecting your blood (including leukaemia) which is now in remission
• have had a stroke or heart attack, or if you have heart problems
• disease of your arteries
Your doctor will decide if you can use Levosert® if you have or have had some of the above conditions.
You must also tell your doctor if any of these conditions occur for the first time whilst you have Levosert® in place.
You must see a doctor or nurse as soon as possible if you develop painful swelling in your leg, sudden chest pain or difficulty in breathing as these may be a sign of a blood clot. It is important that any blood clots are treated promptly.
You must also see a doctor without delay if you develop persistent lower abdominal pain, fever, pain during sexual intercourse or abnormal bleeding. If you get severe pain or fever shortly after Levosert® has been inserted, you may have a severe infection which must be treated immediately.
Levosert9 and smoking Women are advised to give up smoking. Smoking increases the risk of developing a heart attack, stroke, or blood clot.
Tell your doctor if you are taking or have recently taken or might take any other medicines including medicines obtained without a prescription.
The effect of hormonal contraceptives such as Levosert® may be reduced by medicines that increase the amounts of enzymes made by the liver. Please tell your doctor or nurse if you are taking:
• Phenobarbital, primidone, phenytoin or carbamazepine (to treat epilepsy)
• Griseofulvin (an antifungal)
• Rifampicin or rifabutin (antibiotics)
• Nevirapine or efavirenz (for HIV)
Pregnancy, breast-feeding and fertility
Can I become pregnant whilst using Levosert9?
It is very rare for women to become pregnant with Levosert® in place.
Missing a period may not mean that you are pregnant. Some women may not have periods whilst using the system.
If you have not had a period for 6 weeks then consider having a pregnancy test. If this is negative there is no need to carry out another test, unless you have other signs of pregnancy, e.g. sickness, tiredness or breast tenderness.
If you become pregnant with Levosert® in place, please contact your doctor as soon as possible so that ectopic pregnancy can be excluded and Levosert® removed to reduce the risk of spontaneous miscarriage.
What if I want a baby?
If you want a baby, ask your doctor to remove Levosert®. Your usual level of fertility will return very quickly after the system is removed.
Can I breast feed while using Levosert9?
Very small amounts of the hormone in Levosert® are found in breast milk but the levels are lower than with any other hormonal contraceptive method.
No risk for the new-born is to be expected. If you want to breast feed your baby, you should discuss this with your doctor.
There are no known effects on the ability to drive or use machines.
Levosert® contains barium sulphate
The T-frame of Levosert® contains barium sulphate so that it can be seen on X-rays.
Only a doctor or specially trained nurse can fit the system (see special instructions for insertion in the package).
They will:
• give you a pelvic examination to find the position and size of your womb
• place a speculum (an instrument to help the doctor see the cervix) into your vagina
• clean your vagina and cervix
• place a thin flexible tube containing the device into your vagina and then through the cervix into the womb. At this point you might feel a little discomfort
• withdraw the tube leaving the device in place
• trim the threads to a suitable length for easy removal.
During insertion procedure, a slight discomfort might be encountered. Inform your doctor about any pain you feel.
The device should be inserted either during your period or within seven days from the beginning of your period. If you already have the device and it is time to replace it with a new one, you do not need to wait until your period.
If you have just had a baby, you should wait at least 6 weeks before having Levosert® fitted. Levosert® can sometimes be fitted immediately after you have had an abortion, provided that you have no genital infections.
If you have fits (epilepsy), teii the doctor or nurse fitting the Levosert® because, although rare, a seizure (fit) can occur during insertion.
Some women might feel faint after the device is fitted. This is normal and your doctor will tell you to rest for a while.
In very rare cases during fitting, part or all of the device could penetrate the wall of the womb.
If this happens the device is removed.
How quickly should Levosert® work?
Contraception:
You are protected from pregnancy as soon as the system is fitted. The possibility of becoming pregnant is approximately 2 in 1,000 per year. The failure rate may increase in case of Levosert® coming out by itself or perforation.
Heavy menstrual bleeding: Levosert® usually achieves a significant reduction in menstrual blood loss in 3 to 6 months of treatment.
How often should I have the system checked?
You should have the system checked usually 6 weeks after it is fitted, again at 12 months and then once a year until it is removed.
What happens if the system comes out by itself?
If the system comes out either completely or partially, you may not be protected against pregnancy. It is rare but possible for this to happen without you noticing during your menstrual period. An unusual increase in the amount of bleeding during your period might be a sign that this has happened. Tell your doctor or clinic if there are any unexpected changes in your bleeding pattern.
How can I tell whether the system is in place?
After each menstrual period, you can feel for the two thin threads attached to the lower end of the system. Your doctor will show you how to do this.
Do not pull the threads because you may accidentally pull it out. If you cannot feel the threads, go to your doctor.
You should also go to your doctor if you can feel the lower end of the device itself or you or your partner feel pain or discomfort during sexual intercourse.
If you stop taking Levosert®
Your doctor can remove the system at any time. The removal is very easy. Unless you plan to have a new system or an intrauterine device fitted immediately, it is important to use another form of contraception in the week leading up to the removal. Intercourse during this week could lead to pregnancy after Levosert® is removed.
How will Levosert® affect my periods?
Levosert® will affect your menstrual cycle. You might experience spotting, shorter or longer periods, painful periods, lighter periods or no periods at all.
For all users of Levosert®:
Many women have spotting (a small amount of blood loss) for the first 3-6 months after the system is fitted. Others will have prolonged or heavy bleeding. You may have an increase in bleeding however, usually in the first 2 to 3 months, before a reduction in blood loss is achieved. Overall you are likely to have fewer days bleeding in each month and you might eventually have no periods at all. This is due to the effect of the hormone (levonorgestrel) on the lining of the womb.
If you have had Levosert® fitted for heavy menstrual bleeding:
Levosert® usually achieves a significant reduction in menstrual blood loss in 3 to 6 months of treatment. You may have an increase in bleeding however, usually in the first 2 to 3 months, before a reduction in blood loss is achieved.
If a significant reduction in blood loss is not achieved after 3 to 6 months, alternative treatments should be considered.
If you have had Levosert® fitted for quite a long time and then start to have bleeding problems, contact your doctor or clinic for advice.
If you have any further questions on the use of this medicine, ask your doctor.
Moreover, see a doctor as soon as possible if you get:
• painful swelling in your leg
• sudden chest pain
• difficulty breathing Because these may be signs of a blood clot.
Perforation
In rare cases it is possible that the womb or the neck of the womb is pierced, most commonly during insertion of Levosert®. This may be associated with severe pain and continued bleeding.
If perforation is suspected the system should be removed as soon as possible. Contact your doctor immediately if you cannot feel the threads.
The risk of perforation may be increased in insertions following delivery, in lactating women and in women with a fixed retroverted (bent backwards) uterus.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
With Levosert®, side effects are most common during the first months after the system is fitted and decrease as time goes on.
Very common (affects more than 1 in 10 women) side effects can include:
• menstrual changes. You might experience spotting, shorter or longer periods, painful periods. Though Levosert® usually achieves a significant reduction in menstrual blood loss in 3 to 6 months of treatment, you may have an increase in bleeding, usually in the first 2 to 3 months, before a reduction in blood loss is achieved.
Periods can totally disappear. If a significant reduction in blood loss is not achieved after 3 to 6 months, alternative treatments should be considered.
• ovarian cysts. They are fluid-filled sacs in the ovary.
Common (may affect up to 1 in 10 women) side effects can include:
• bloating or swelling of your legs or ankles;
• weight gain;
• depression, nervousness or other mood changes;
• headache;
• abdominal, pelvic or back pain;
• feeling sick (nausea);
• spots (acne);
• painful periods;
• increased vaginal discharge;
• inflammation of the neck of the womb (cervicitis);
• tender, painful breasts; or
• Levosert® coming out by itself.
Uncommon (may affect up to 1 in 100 women) side effects can include:
• genital infections that may cause: vaginal itching; pain on passing urine; or lower abdominal (tummy) pain from inflammation of the womb, ovaries or fallopian tubes;
• increased growth of hair on the face and body;
• hair loss; or
• itchy skin (pruritus).
Rare (may affect up to 1 in 1000 women) side effects can include:
• reduced sex drive;
• migraine;
• bloated abdomen;
• rashes, itching, eczema; or
• the wall of the womb torn when the Levosert® is fitted.
Ovarian cysts and pelvic inflammatory disease have been reported. So tell your doctor if you have lower abdominal pain or if you experience painful or difficult sex. This is important as pelvic infections can reduce your chances of having a baby and can increase the risk of ectopic pregnancy (development of a foetus outside the womb).
Ectopic pregnancy is possible with Levosert® but highly unlikely. The risk of this happening is lower than for women using no contraception or a copper intrauterine device.
You should tell your doctor if you have lower abdominal (tummy) pain especially if you also have a fever or have missed a period or have unexpected bleeding. This might be a sign of ectopic pregnancy.
If you think you are reacting badly to Levosert® or are having any other problems, please tell your doctor or clinic.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
Store in the original package. Do not open the Levosert® pack. Only your doctor or clinic should do this.
This medicinal product does not require any special temperature storage conditions.
Keep this medicine out of the sight and reach of children.
Do not use the device after the expiry date which is stated on the label and the outer pack after EXP. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
• Levosert® consists of a small T-shaped frame made from a plastic called polyethylene. This carries 52 mg of levonorgestrel, the active substance, a hormone used in many contraceptive pills and hormone replacement therapy preparations. The hormone is contained within a substance called polydimethylsiloxane.
This is surrounded by a membrane (skin) also made of polydimethylsiloxane.
• The T-shaped frame also contains barium sulphate so that it can be seen on X-rays.
• This structure provides a device for releasing the hormone gradually into the uterus (womb).
• There are two fine threads, made of polyethylene and copper phtalocyanine blue, attached to the bottom of the frame. These allow easy removal and allow you or your doctor to check that the device is in place.
What Levosert® looks like and contents of the pack
Each pack contains one Levosert®.
Marketing Authorisation Holder
Actavis Group PTC ehf. Reykjavikurvegi 75-78 220 HafnarfjorSur Iceland
Odyssea Pharma SA Rue du Travail 15 4450 Grace Hollogne Belgium
Gedeon Richter Pic.
Gyomroi ut 19-21 1103 Budapest Hungary
This leaflet was last revised in August 2014
The following information is intended for medical or healthcare professionals only
See special instruction leaflet enclosed in the pack. AAAG7629
co
o
o
•r—I
o
o
03
y
*6
<v
T3
4)
T3
C
4) .
.et
oo O
o g
■§.2
r oo C oo >r
O 'te
•s a
p ^ 2 ££ o n3 0) a)£
£ i-
E— O
Prescriber Check List
Ask yourself the following questions before prescribing/inserting
Levosert®:
• I have checked that the patient's needs meet the indications of contraception or heavy menstrual bleeding?
• I have checked that the patient doesn't reach any contra-indication:
□ Known or suspected pregnancy
□ Current or recurrent pelvic inflammatory disease
□ Current genital infection
□ Postpartum endometritis
□ Infected abortion during the past three months
□ Cervicitis, Cervical dysplasia
□ Suspected or confirmed uterine or cervical malignancy
□ Liver tumour or other acute or severe liver disease
□ Congenital or acquired abnormality of the uterus including fibroids if they distort the uterine cavity
□ Undiagnosed abnormal genital bleeding
□ Conditions associated with increased susceptibility to infections
□ Active or previous severe arterial disease, such as stroke or myocardial infarction
□ Current or suspected hormone dependent tumours such as breast cancer
□ Hypersensitivity to the active constituents of the preparation
□ Acute malignancies affecting the blood or leukaemias except when in remission
□ Recent trophoblastic disease while hCG levels remain elevated
0
</>
c
o
+-»
u
3
</>
e
rji
C
■i—*
c
(C
X
T3
C
(C
<D
</>
»-
O
Conditions for use
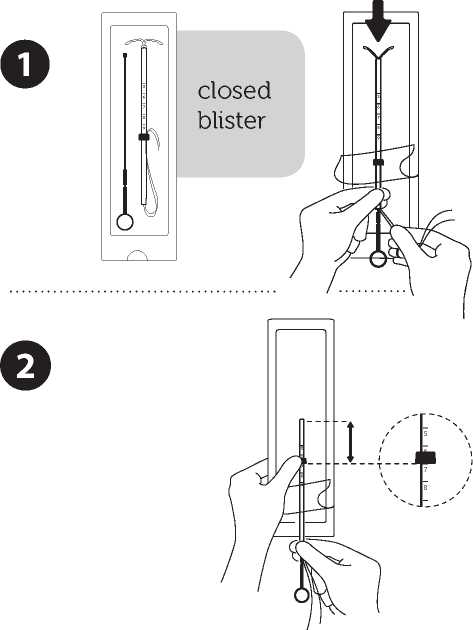
1. In women of fertile age, Levosert1® is inserted within seven days of the onset of menstruation. It can be replaced by a new system at any time of the cycle.
2. It is recommended that Levosert® should only be inserted by physicians/health care professionals who have undergone sufficient training and have read carefully these instructions before Levosert® insertion.
3. Levosert'Us supplied in a sterile pack. Do not use if the inner package is damaged or open.
4. Determine the position (anteversion, retroversion) and size of the uterus by a gynaecological examination. Exclude pregnancy and contra-indications.
5. Place a speculum, use appropriate antiseptic solution to clean the vagina and cervix.
6. Use cervical dilators if cervical stenosis is diagnosed. Do not force to overcome resistance.
7. Grasp the cervix with a Tenaculum forceps and apply a gentle traction in order to straighten alignment of the cervical canal and uterine cavity.
8. Determine the uterine depth by hysterometry. If uterine depth is < 5.5 cm discontinue the procedure.
OO©©0®@0©©
hysterometer value
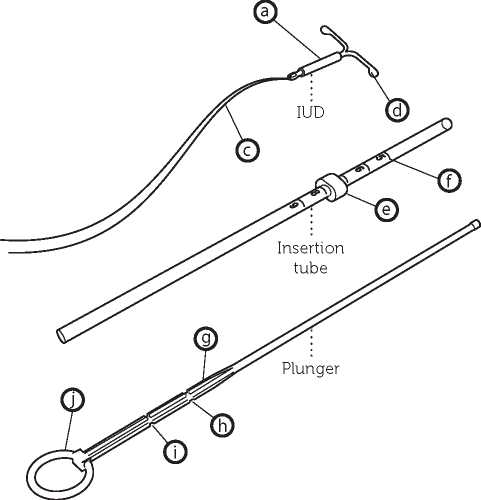
Description
Levonorgestrel containing cylinder Lateral arms Threads Knobs Flange Scale
Thickened mark First indent Second indent Ring
Preparation for insertion
Introduce the plunger and the IUD in the insertion tube
Partly open the blister (about 1/3 from the bottom) and introduce the plunger in the insertion tube Extricate the threads from the flange Pull the thread to introduce the IUD into the tube Tlae arms of the IUD must stay in an horizontal plan, parallel to the flat side cl the flange
Position the lower edge of the flange at the sounded value
Position the blue flange so as the lower edge of the flange indicates the value found by hysterometry. The flat sides of the flange must always remain parallel to the arms. This will allow the arms to open correctly in the uterine cavity.
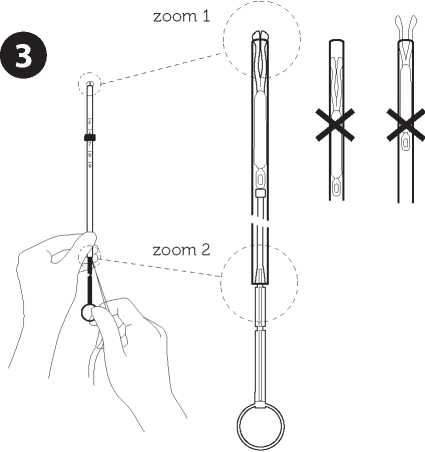
Adjust the position of the IUD in the insertion tube
Hold the plunger firmly while pulling the thread and moving the tube to adjust the IUD s position. The knobs of the lateral arms must be closely opposed to each other, slightly above the upper extremity of the insertion tube (see zoom 1) and the distal edge of the tube must be aligned with the first indent of the plunger (see zoom 2). If the tube is not aligned with the first indent of the plunger you must pull the thread more firmly.
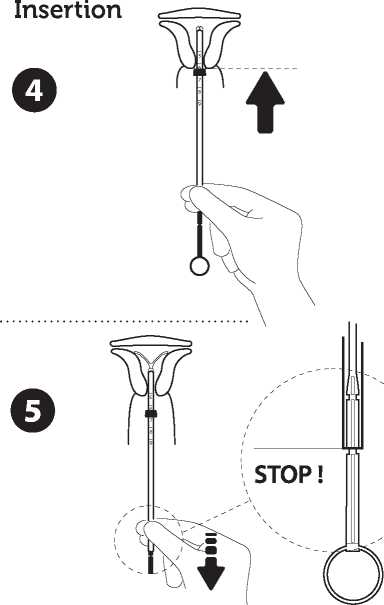
Introduce the device in the cervical canal until the blue flange is in contact with the cervix
Take the whole device out of the blister, by holding firmly the plunger and tube together in the correctly adjusted position. Introduce the assembly into the cervical canal until the blue flange is in contact with the cervix.
Release the arms of the intrauterine device
Hold the plunger, release the thread and pull the insertion tube down until its lower extremity reaches the second indent of the plunger
Push the IUD against the fundus
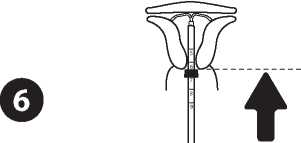
To position the IUD in the uterine cavity, push the insertion tube simultaneously with the plunger, until the blue flange is again in contact with the cervix. Levosert® is then correctly placed in the uterine cavity.
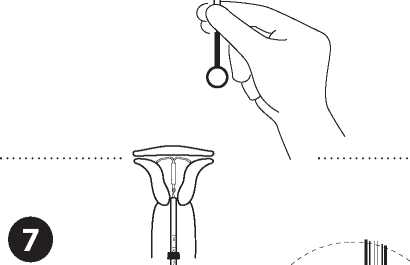
Release the IUD from the tube into the uterine cavity
Without moving the plunger, pull the insertion tube eleven to the ring of the plunger A slight resistance marks the passage of the bulge of the plunger Nevertheless pull eleven the tube to the ring of the plunger Levosert® is then released completely from the insertion tube
Remove sequentially the inserter components and cut the threads
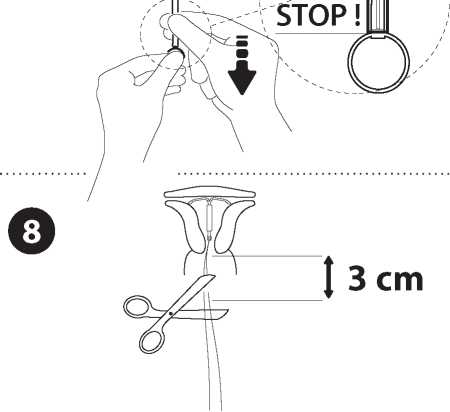
Retrieve seguentially, first, the plunger, then the insertion tube Cut the threads at around 3 ern from the cervix
IMPORTANT!
In case of difficult insertion and/or exceptional pain or bleeding during or after insertion, physical examination and ultrasound should be performed immediately to exclude perforation of the uterine body or cervix. If necessary remove the system and insert a new, sterile system.
Please report to our pharmacovigilance department any case of uterine perforation or insertion difficulties: Actavis Group PTC ehf. E-mail: medinfo@actavis.co.uk Phone: 01271385257.
Instructions for use and handling was last revised in Aug 2014
AAAG7630