Lidocaine Hcl 5%W/V And Phenylephrine Hcl 0.5%W/V Topical Solution

Bampton Road, Harold Hill, Romford RM3 8UG, United Kingdom
Aurum
PACKAGE LEAFLET: INFORMATION FOR THE USER
Lidocaine Hydrochloride 5 % w/v and Phenylephrine Hydrochloride 0.5 % w/v Topical Solution
In this leaflet:
1. What Lidocaine and Phenylephrine Topical Solution is and what it is used for
2. Before lidocaine and Phenylephrine topical solution is used
3. How will lidocaine and Phenylephrine topical solution be used?
4. Possible side-effects
5. storing lidocaine and Phenylephrine topical solution
6. further information.
1. What Lidocaine and Phenylephrine Topical Solution is and what it is used for
the name of this product is lidocaine hydrochloride 5% w/v and Phenylephrine hydrochloride 0.5% w/v topical solution. the active ingredients are local anaesthetic (lidocaine) which causes numbness and a sympathomimetic drug (phenylephrine) that reduces bleeding.
Lidocaine and phenylephrine topical solution is applied to the area inside your nose and throat to numb the area, and reduce bleeding prior to performing certain surgical procedures.
it is also used to aid in the removal of foreign bodies from the nose.
2. Before Lidocaine and Phenylephrine Topical Solution is used.
lidocaine and Phenylephrine topical solution should not be given to you if:
• you are allergic (hypersensitive) to Lidocaine, Phenylephrine or any of the ingredients of this medicine (listed in section 6)
• you are taking monoamine oxidase inhibitors, (drugs used to treat depression) or have taken them within the last two weeks.
• you are taking a type of medicine known as sympathomimetics. this can include medicines used to treat asthma, colds and flu, glaucoma, heart problems or problems with your blood pressure and premature labour
• you are sensitive to some local anaesthetics similar to Lidocaine
• you are sensitive to vasoconstrictors; such as phenylephrine
• you have a low amount of blood in your body (hypovolaemia)
• you have some problems with electrical signals in your heart e.g. you need a pacemaker (a device that regulates heart beats)
• you are pregnant or breast-feeding
• you suffer from high blood pressure
• you suffer from a type of heart disease known as acute ischaemic heart disease
• you suffer from problems with your thyroid gland (thyrotoxicosis)
• you suffer from glaucoma (increased pressure in the eyeball)
• you have difficulty passing urine.
Take special care with Lidocaine and Phenylephrine Topical Solution
Tell your doctor if:
• you have any heart disease
• you suffer from diabetes
• you suffer from low blood oxygen level or a high blood carbon dioxide level (for example you suffer from any lung disease)
• you suffer from porphyria (a hereditary disorder that effects some enzymes in the blood and liver)
• you have an overactive thyroid gland
• you suffer from fits
• you have liver or kidney problems
• you are suffering from severe shock (sweating, feel unwell, feeling your heartbeat, fainting)
• you have any cut or sore area in your nose or throat
• you suffer from angina.
Taking other medicines:
Please tell your doctor if you are taking any of the following medicines.
• beta-blockers (drugs used in the treatment of heart problems or high blood pressure)
• debrisoquine, reserpine, methyldopa and guanethide (used to treat high blood pressure)
• quinupristin/dalfopristin (used to treat bacterial infections resistant to antibiotics)
• diuretics (drugs used to treat difficulty in passing urine and water retention)
• reboxetine (used to treat depression)
• suxamethonium (a muscle relaxant)
• ergot alkaloids (used to treat migraine)
• doxapram (used to treat severe breathing difficulties)
• oxytocin (used to induce labour)
• anaesthetics such as chloroform, halothane or isoflurane
• phenothiazines (used to treat certain mental disorders)
• drugs used to treat depression known as tricyclic antidepressants. these include amitriptyline, clomipramine and imipramine
• monoamine oxidase inhibitors (MAOIs) (drugs used to treat depression). You Must Not use lidocaine and Phenylephrine Topical Solution if you are taking MAOIs or if you have taken them within the last two weeks
• drugs for treatment of heart arrhythmias (irregular heart beat), such as lidocaine, digoxin or tocainide.
if you have any doubts about whether this medicine should be administered to you, discuss this more fully with your doctor or nurse.
Pregnancy and breast feeding:
Lidocaine and Phenylephrine Topical solution should not be administered to those who are pregnant or breast-feeding.
Effects on the ability to drive and use machinery:
It is unlikely that after using Lidocaine and Phenylephrine Topical solution, your ability to drive and use machines will be affected. However, if you feel unwell you must speak to your doctor before driving or operating machinery.
3. How will Lidocaine and Phenylephrine Topical Solution be used?
Your doctor or nurse will administer the solution onto the area inside your nose or throat, using the pump spray dispenser supplied with the product.
Dose:
Adults, and children over 12 years: Up to a maximum of 8 sprays in total.
children of 12 years and under: not recommended.
4. Possible side-effects
Like all medicines Lidocaine and Phenylephine Topical Solution can cause side effects, although not everybody gets them.
The anaesthetic effect induced by lidocaine may cause numbness of the tongue and mouth and may interfere with swallowing. Do not eat or drink until the effect has worn off.
If used on damaged or inflamed skin Lidocaine and Phenylephrine Topical solution may cause the following effects:
Breathing difficulties, fits, coma, heart attack, chest pain, bradycardia (slow heart rate), cardiac arrhythmias (irregular heart beat) and low blood pressure (with dizziness, fainting and flushing) or high blood pressure. These are all very serious side effects and are very rare.
if any of the above happen, tell your doctor immediately or go to the casualty department at your nearest hospital as you may need urgent medical attention.
Tell your doctor or go to the casualty department at your nearest hospital if you notice any of the following:
Difficulty in passing urine (particularly if you have problems with your prostate), blurred vision, dizziness, drowsiness, irritability, extremely high temperature, muscle twitching and shaking. You may need medical attention. These serious side effects are rare.
Tell your doctor if you notice any of the following:
Excessive saliva, headache, reduced appetite, sweating and weakness, difficulty in sleeping, feeling sick or being sick, anxiety, confusion, excitement, fear, nervousness, restlessness, fits and tinnitus. These are all mild side effects of Lidocaine and Phenylephrine Topical solution.
If you experience any side effects or think you may be reacting badly in any way, inform your doctor, or nurse immediately.
5. Storing Lidocaine and Phenylephrine Topical Solution
You should not be given the solution if the expiry date on the label has passed or if it shows signs of deterioration. The doctor or nurse will check that the expiry date on the label has not passed and that the solution does not show signs of deterioration.
Do not store the solution above 25°c.
Keep all medicines out of the sight and reach of children. Keep in the original container.
6. Further information
What Lidocaine and Phenylephrine Topical Solution contains:
The active ingredients in Lidocaine Hydrochloride 5% w/v and Phenylephrine Hydrochloride 0.5% w/v Topical solution are Lidocaine Hydrochloride BP and Phenylephrine Hydrochloride BP. Each bottle contains 2.5ml of solution containing 50mg/ ml of lidocaine hydrochloride and 5mg/ml of phenylephrine hydrochloride in Water for Injections BP, with sodium hydroxide and hydrochloric acid to adjust the pH.
What Lidocaine and Phenylephrine Topical Solution looks like and contents of the pack:
Lidocaine and phenylephrine topical solution is licensed to be available in one bottle containing 2.5ml of solution, in a single box. Each 2.5ml of solution contains 125mg of Lidocaine Hydrochloride BP and 12.5mg of Phenylephrine Hydrochloride BP respectively.
Marketing Authorisation Holder: Aurum Pharmaceuticals Ltd., Bampton Road, Romford, RM3 8UG, United Kingdom.
Manufacturers: Pharmacy Production Unit, Queens Hospital, Belvedere Road, Burton-on-Trent, staffordshire, DE13 0rB,
United Kingdom.
Martindale Pharmaceuticals, Bampton Road, Romford, RM3 8UG, England.
Product Licence No.: PL 12064/0027 Date of Revision: November 2010
If this leaflet is difficult to see or read, please contact the marketing authorisation holder for help.
a
o
O'
_-r£
package leaflet: INFoRMATIQN for the user D01546
■Q f
1. Immediately before use. remove the screw cap and rubber stopper from the bottle.
2. Screw the pump onto the bottle.
3. Push the actuator onto the top of the pump.
4. Press down on the pump-actuator 3 times to prime the pump before use.
5. The pump spray is now ready for use. Refer to the Patient Information Leaflet or Summary of Product Characteristics for dosing instructions.
Lidocaine Hydrochloride 5 % w/v and Phenylephrine Hydrochloride 0.5 % w/v Topical Solution
User Guide
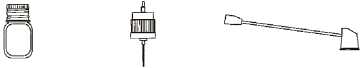
Bottle Pump Actuator
|
rr~ | |
|
vv- |
=J |
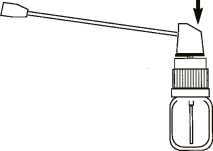
Instructions For Assembly Of Pump + Actuator To Bottle.
Priming the Pump.
While holding the bottle upright, the pump should be activated three times before use to prime it.
Other Information.
The pump dispenser will deliver a dose volume of 130pl in each spray. Thus, each spray is equivalent to 6.5mg of Lidocaine Hydrochloride BP and 0.65mg of Phenylephrine Hydrochloride BP.
Always hold the bottle upright to ensure dip-tube is immersed in the solution at its tip.
This product is sterile until opened, and contains no preservative.
Use once for one patient only and discard the bottle and any remaining solution in an appropriate manner. Do not use if packaging is damaged.
Keep out of the reach of children.
This product also contains Water for Injections, with sodium Hydroxide and /or Hydrochloric Acid to adjust pH.
MA Number/Holder
12064/0027 Aurum Pharmaceuticals Ltd., Bampton Road, Romford, RM3 8UG, England.
For further information contact:
Aurum Pharmaceuticals Ltd.
Bampton Road, Romford
RM3 8UG
England.
a
o
Bampton Road, Harold Hill, Romford £
RM3 8UG, United Kingdom
Aurum
100mm Measurement Verification Bar