Lifsar 50 Microgram/500 Microgram Per Metered Dose Inhalation Powder
Package leaflet: Information for the user
Lifsar ® 50 microgram/500 microgram per metered dose Inhalation powder
Salmeterol/fluticasone propionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Lifsar is and what it is used for
2. What you need to know before you use Lifsar
3. How to use Lifsar
4. Possible side effects
5. How to store Lifsar
6. Contents of the pack and other information
1. What Lifsar is and what it is used for
The name of your medicine is Lifsar ® 50 microgram/500 microgram per metered dose Inhalation powder (called Lifsar throughout this leaflet). Lifsar is an inhaler that contains two active ingredients, salmeterol and fluticasone propionate.
Salmeterol is a long-acting bronchodilator. Bronchodilators help the airways in the lungs to stay open. This makes it easier for air to get in and out. The effects last for at least 12 hours. Fluticasone propionate is a corticosteroid which reduces swelling and irritation in the lungs.
The doctor has prescribed this medicine to help to prevent breathing problems associated with Chronic Obstructive Pulmonary Disease (COPD). Lifsar reduces the number of flare ups of symptoms related to COPD.
You must use Lifsar every day, as prescribed by your doctor. This will ensure that the medicine works properly in controlling these symptoms.
Lifsar helps to stop breathlessness and wheeziness coming on. However, Lifsar should not be used to relieve a sudden attack of breathlessness of wheezing. If this happens you need to use a fast-acting “reliever” (“rescue”) inhaler, such as salbutaniol. You should always have your fast-acting “rescue” inhaler with you.
Lifsar is intended for use in adults with COPD 18 years of age and older only.
Not for use in asthma.
2. What you need to know before you use Lifsar Do not use Lifsar
• If you are allergic to salmeterol, fluticasone propionate or the other ingredient in this medicine (listed in section 6).
Warnings and precautions
Your doctor will supervise your treatment more closely if you have or have ever had:
• Heart disease, including irregular or fast heartbeat.
• Overactive thyroid gland.
• High blood pressure.
• Diabetes mellitus (Lifsar may increase your blood sugar).
• Low potassium in your blood.
• Tuberculosis (TB) now or in the past, or other lung infection.
Talk to your doctor before using Lifsar if any of the above applies to you.
Children and adolescents
Do not give this medicine to children and adolescents less than 18 years of age, because it may not be safe or effective. Lifsar is intended for use in adults 18 years of age and older only.
Other medicines and Lifsar
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, before you start using Lifsar. This includes other medicines for COPD, other inhalers or any medicines obtained without a prescription. This is because Lifsar may not be suitable to be taken with some other medicines.
In particular tell your doctor if you are taking any of the following:
• Beta blockers (such as atenolol, propranolol, sotalol). Beta blockers are mostly used for high blood pressure and heart conditions.
• Medicines to treat infections caused by viruses and fungi (such as ritonavir, ketoconazole, itraconazole and erythromycin). Some of these medicines may increase the amount of fluticasone propionate or salmeterol in your body, which can increase your risk of experiencing side effects, including irregular heartbeats, or may make side effects worse.
• Corticosteroids (taken by mouth or by injection). If you have used these medicines recently, this might increase the risk of Lifsar affecting the function of your adrenal gland.
• Diuretics, also known as ‘water tablets’ used to treat high blood pressure.
• Other bronchodilators (such as salbutamol).
• Xanthine medicines. These are often used to treat asthma.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Your doctor will assess whether you can take this medicine or not.
Driving and using machines
Lifsar is unlikely to affect your ability to drive or use machines.
Lifsar contains lactose monohydrate (a type of sugar)
Lifsar contains up to 7 milligrams of lactose monohydrate in each dose.
The amount of lactose in this medicine does not normally cause problems in people who are lactose intolerant. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to use Lifsar
Always use this medicine exactly as your doctor has told you. Check with your doctor, pharmacist or nurse if you are not sure.
• Inhalation use.
• Use Lifsar every day, until your doctor advises you to stop.
• Do not take more than the recommended dose.
• Do not stop taking Lifsar or reduce the dose of Lifsar without talking to your doctor first.
• Lifsar should be inhaled through the mouth into the lungs.
Adults
The usual dose is one inhalation, twice a day.
It is very important to follow your doctor’s instructions on how many inhalations to take and how often to take your medicine.
Use in children and adolescents
This medicine is not for use in children and adolescents less than 18 years of age, because it may not be safe or effective.
If your breathing gets worse or is not well controlled even if Lifsar is used regularly as prescribed by your doctor, tell your doctor straight away. You may feel more wheezy, your chest feels tight more often or you may need more of your fast-acting ‘reliever’ medicine). If any of these happen, you should continue to take Lifsar but do not increase the number of inhalations you take. Your chest condition could be getting worse and you could become seriously ill. See your doctor straight away as you may need additional treatment.
How to use your inhaler
Your doctor, nurse or pharmacist should show you how to use your inhaler. They should check on how you use it from time to time. If you do not use the inhaler properly or as prescribed, the medicine might not help your COPD and your symptoms as it should.
Lifsar releases a powder which is inhaled into the lungs.
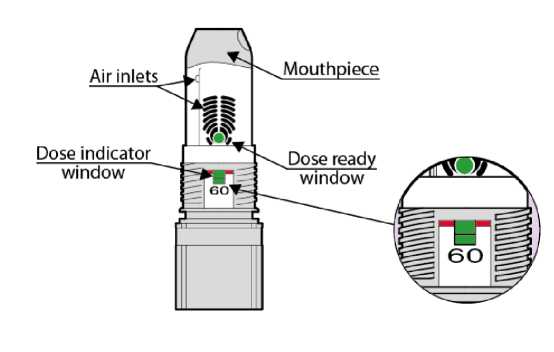
Note: The number shown at the dose indicator window refers to the initial number of inhalations (60) in your device and it doesn't change even if the device is empty. The green colour in the dose indicator window will show you approximately how many inhalations are left (see below in text the section “When you should replace your inhaler”).
The basic principles for using Lifsar are
1. OPEN: Remove the white cover.
2. INHALE: Place your lips around the mouthpiece and inhale deeply.
3. CLOSE TO CLICK: Replace the cover fully.
Please carefully read the instructions for use below before using Lifsar for the first time.
Follow the instructions below every time you need to take an inhalation.
Instructions for use
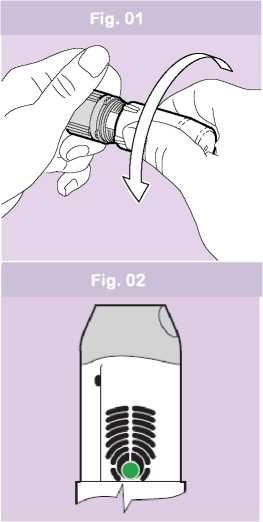
Fig. 03
1. OPEN
• Hold the device with two hands: one hand on the grey base and the other on the protective white cover. You can hold your device in any position.
• Remove the white protective cover from the grey base by twisting both in opposite directions. (Fig. 01). You will feel a small resistance when the cover is half opened.
• The green colour in the dose ready window confirms to you that your inhaler is ready for use (Fig. 02).
2. INHALE
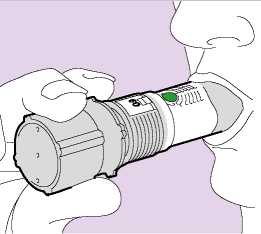
• Hold the inhaler firmly by the grey base, away from your mouth. Breathe out slowly as far as is comfortable.
Do not breathe through the inhaler.
• Place your lips around the grey mouthpiece (Fig. 03). Do not cover any of the air inlets with your lips. Do not chew or bite the mouthpiece.
• Breathe in as deeply and as hard as you can through your mouth (not through your nose).
• Do not stop inhaling when you hear a soft "plopping" sound. The soft “plopping” sound during the inhalation process indicates that the dose has been released.
• Remove the inhaler from your mouth and hold your breath for an additional 5-10 seconds or as long as is comfortable. Breathe out slowly through your nose and return to normal breathing.
• The green colour in the dose ready window will now have disappeared. This confirms that the dose has been successfully delivered (Fig. 04).
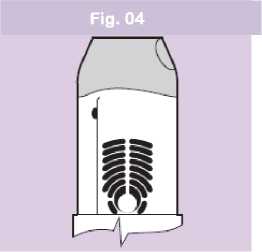
Note: The amount of medicine you inhale is very small and you may not be able to taste it. However, you can be confident that you have taken the dose and the medicine is in your lungs if the green colour in the dose ready window has disappeared.
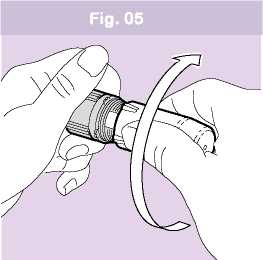
3. CLOSE TO CLICK
• Twist the white protective cover back firmly onto the grey base until it clicks (Fig. 05).
• It is important that you fully rotate the cover until you hear the click as this loads your next dose of medicine. The alignment lines on the cover and base should match.
If you need more than one dose
If the doctor told you that you have to take more than one inhalation, repeat the 3 steps above.
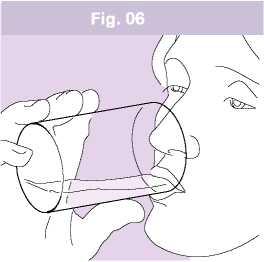
After you have taken your dose rinse your mouth with water (Fig. 06) and/or brush your teeth. Do not swallow the water you used for rinsing your mouth or brushing your teeth, spit it out.
This will reduce the risk of you developing oropharyngeal candidiasis (Thrush, a fungal infection, in your mouth and/or throat) and developing hoarseness.
The number of initial inhalations (60) in your device is shown in the dose indicator window (Fig. 07).
Fig. 07
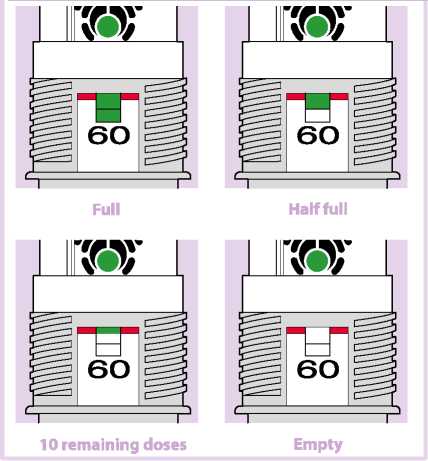
This number doesn't change even if the device is empty.
The green colour in the dose indicator window will show you approximately how many inhalations of the medicine are left in the device. Hold your inhaler upright at eye level to check how much is left in the container.
When the green colour reaches the level of the red line, it means you have approximately 10 inhalations left in the device. (Fig. 07 - 10 remaining doses). Continue using the inhaler, but see your doctor as soon as possible to get a new prescription.
When the green indicator is no longer visible, there are no inhalations left and your current inhaler must be replaced with a new one (Fig. 07 - Empty).
Cleaning your inhaler
• If the mouthpiece gets dirty, you can clean it by wiping it with a dry, clean tissue. Do not use water or other liquids to clean the mouthpiece.
• Always close the white protective cover of the inhaler when you are not using it.
• Protect the inhaler against moisture.
Other information about your inhaler
• The white protective cover will still twist and “click” even when the inhaler is empty.
• The sound you hear as you shake the inhaler is produced by a drying agent and not
by the medicine. The sound does not tell you how much medicine is left in your inhaler.
• If you load the inhaler more than once, by mistake, before taking your dose, you will still only receive one dose.
• If the device is dropped without the cap, then the cap should be replaced before the next dose is taken.
If you use more Lifsar than you should
It is important to use the inhaler as instructed. If you accidentally take a larger dose than recommended, talk to your doctor, pharmacist or nurse. You may notice your heart beating faster than usual and you may feel shaky. You may also have dizziness, a headache, muscle weakness and aching joints.
If you have used larger doses for a long period of time, you should talk to your
doctor, pharmacist or nurse for advice. This is because larger doses of this medicine may reduce
the amount of steroid hormones produced by your adrenal gland.
If you forget to use Lifsar
If you forget to use your inhaler, take your next inhalation when it is due.
Do not take a double inhalation to make up for a forgotten one.
If you stop using Lifsar
It is very important you use the inhaler every day, as the doctor recommended.
Keep taking your medicine until your doctor tells you to stop. Do not stop or suddenly reduce your dose of Lifsar. This can make your breathing worse. Very rarely, if you suddenly stop using your inhaler or suddenly reduce the dose, this can cause you to have problems with your adrenal gland (adrenal insufficiency) and you may experience side effects. These may include any of the following:
• Stomach pain.
• Tiredness and loss of appetite, feeling sick.
• Sickness and diarrhoea.
• Weight loss.
• Headache or drowsiness.
• Low levels of sugar in your blood.
• Low blood pressure and seizures (fits).
When your body is under stress such as from fever, trauma (such as a car accident), infection, or surgery, adrenal insufficiency can get worse and you may have any of the side effects listed above. To prevent these symptoms occurring, your doctor may prescribe extra corticosteroids in tablet form (such as prednisolone).
If you do get any of the side effects listed above, or any other side effects, talk to your doctor or pharmacist straight away.
If you have any further questions on the use of this medicine or device, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions: you may notice your breathing suddenly gets worse immediately after using Lifsar. You may be very wheezy and cough or be short of breath. You may also notice itching, a rash (hives) and swelling (usually of the face, lips, tongue, or throat), or you may suddenly feel your heart beating very fast or you feel faint and light headed (which may lead to collapse or loss of consciousness). If you get any of these effects or if they happen suddenly after using Lifsar, stop using Lifsar and tell your doctor straight away. Allergic reactions to Lifsar are uncommon (they affect less than 1 person in 100).
Other side effects are listed below:
Very common (affects more than 1 person in 10):
• Headache - this usually gets better as treatment continues.
• Increased number of colds has been reported.
Common (affects less than 1 person in 10):
• Thrush (sore, creamy-yellow, raised patches) in the mouth and throat. Also sore tongue, throat irritation and hoarse voice. Rinsing your mouth out with water and spitting it out immediately and/or brushing your teeth after taking each dose of your medicine may help. Your doctor may prescribe you an anti-fungal medication to treat the thrush.
• Aching, swollen joints and muscle pain.
• Muscle cramps.
• Pneumonia (in COPD patients) and bronchitis (lung infection). Tell your doctor if you have any of the following while taking Lifsar, they could be symptoms of a lung infection:
- Fever or chills.
- Increased mucus production, change in mucus colour.
- Increased cough or increased breathing difficulties.
• Bruising and fractures.
• Inflammation of sinuses (a feeling of tension or fullness in the nose, cheeks and behind the eyes, sometimes with a throbbing ache).
• A reduction of the amount of potassium in the blood (you may get uneven heartbeats, muscle weakness, cramp).
Uncommon (affects less than 1 person in 100):
• Increase in the amount of sugar (glucose) in your blood (hyperglycaemia). If you have diabetes, more frequent blood sugar monitoring and possibly adjustment of your usual diabetic treatment may be required.
• Cloudy lens in the eye (cataract).
• Very fast heartbeat (tachycardia).
• Feeling shaky (tremor) with fast or uneven heart beat (palpitations) - these are usually harmless and occur less as treatment continues.
• Chest pain.
• Feeling worried (this effect mainly occurs in children).
• Disturbed sleep.
• Allergic skin rash.
Rare (affects less than 1 person in 1000):
• Breathing difficulties or wheezing which worsen immediately after taking Lifsar.
If this happens, stop using Lifsar. Use your fast-acting “reliever” inhaler to help your breathing and tell your doctor immediately.
• Lifsar may affect the normal production of steroid hormones in the body, particularly if you have taken high doses for long periods of time. The effects include:
- Slowing of growth in children and adolescents.
- Thinning of the bones.
- Glaucoma.
- Weight gain.
- Rounded (moon shaped) face (Cushing’s syndrome).
Your doctor will check you regularly for any of the above side effects and will make sure you are taking the lowest dose of salmeterol/fluticasone propionate to control your disease.
• Behavioural changes, such as being unusually active and irritable (these effects mainly occur in children).
• Uneven heartbeats or heart gives an extra beat (arrhythmias). Tell your doctor, but do not stop taking Lifsar unless the doctor tells you to stop.
Frequency not known, but may also occur:
• Depression, aggression (mainly in children).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report any side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Lifsar
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after “EXP”. The expiry date refers to the last day of that month.
Keep the white protective cover tightly closed in order to protect from moisture.
Once opened, use within 30 days.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Lifsar inhaler contains
The active substances are salmeterol (as salmeterol xinafoate) and fluticasone propionate. Each single inhalation provides a delivered dose (the dose leaving the mouthpiece) of 45 micrograms of salmeterol (as salmeterol xinafoate) and 465 micrograms of fluticasone propionate. This corresponds to a metered dose of 50 micrograms of salmeterol (as salmeterol xinafoate) and 500 micrograms of fluticasone propionate.
The other ingredient is lactose monohydrate.
What Lifsar inhaler looks like and contents of the pack
Lifsar is the PulmoJet inhaler containing white powder. The inhaler has two components, a removable white protective cover and the main body. The main body is grey and white, with a grey mouthpiece and a grey base with a purple- or grey-coloured bottom. Lifsar is available in cartons with one inhaler containing 60 inhalations.
Marketing Authorisation Holder
Zentiva, One Onslow Street, Guildford, Surrey, GU1 4YS, UK
Manufacturer(s)
Fisons Limited TA Aventis Pharma (Holmes Chapel)
72 London Road, Holmes Chapel, Crewe, Cheshire CW4 8BE UK
This leaflet was last revised in 07/2016
Other sources of information
Patient information video is available on the website: www.pulmojet.com and by scanning the QR Code included in the PIL and outer carton with a smartphone.
11/11