Loestrin 30
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Loestrin 30
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each Loestrin 30 tablet contains:
Norethisterone acetate Ph Eur 1.5mg
Ethinylestradiol Ph Eur 0.03mg
Excipients with known effect: each tablet contains sunset yellow FCF (E110), 22.01mg of lactose and 38.76mg of sucrose.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Pale green convex film coated tablet.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
For the prevention of pregnancy in women who elect to use oral contraceptives. The efficacy of any contraceptive method, except sterilisation, depends upon the reliability with which it is used. Correct and consistent use of methods can result in lower failure rates.
4.2 Posology and Method of Administration
For oral use.
One Loestrin 30 tablet should be taken daily at approximately the same time of day for three weeks and then an interval of one week allowed before commencing the second course of tablets. Second and subsequent courses should be taken for three weeks with one week without tablets between courses. Thus each new course of
tablets is always started on the same day of the week. It is important that the tablets are taken as directed and should be taken without regard to menstrual bleeding except in the initial cycle.
Pill initiation
Provided that it is reasonably certain that a woman is not pregnant:
• COCs can be started at any time within 5 days of the start of menstrual bleeding without additional contraceptive precautions;
• COCs can be started at any other time in the cycle, or by women who are not menstruating, with additional contraceptive precautions for the first 7 days;
• a switch can be made from other methods of contraception, whether hormonal or not, at any time in the cycle. Additional contraceptive precautions are required for the first 7 days if changing from a non-hormonal method outside the first 5 days of the menstrual cycle.
Post-partum andpost-abortum use
After pregnancy combined oral contraception can be started in non-lactating women 21 days after a vaginal delivery, provided that the patient is fully ambulant and there are no puerperal complications.
If the pill is started later than 21 days after delivery, then barriers and spermicides should be used until oral contraception is started and for the first 7 days of pill-taking. If unprotected intercourse has taken place after 21 days post-partum, then oral contraception should not be started until the first menstrual bleed after childbirth.
After a miscarriage or abortion, oral contraceptives may be started immediately.
Missed pills
• If one pill is missed during weeks 1, 2 or 3, or if a pack is started 1 day late, the pill should be taken as soon as possible and no additional contraceptive precautions need be taken.
• If more than one pill is missed during weeks 1, 2 or 3, or if a pack is started more than 1 day late, a pill should be taken immediately, daily pills should be continued and additional contraceptive precautions should be taken for the next 7 days;
• if the pills are missed in the first week and unprotected sex has taken place, the woman should discuss with a healthcare professional the use of emergency contraception;
• if the pills are missed in the third week, the current course of pills should be completed and the next pack should be started immediately.
Vomiting or diarrhoea may reduce efficacy by preventing full absorption. Barriers and spermicides should therefore be used during and for 7 days after recovery and if these 7 days overrun the end of a pack, the next pack should be started without a break. In this case, a withdrawal bleed should not be expected until the end of the second pack. If the patient does not have a withdrawal bleed at the end of the second pack, she must return to the doctor to exclude the possibility of pregnancy.
4.3 Contraindications
1) Known or suspected pregnancy and lactation.
2) History of confirmed venous thromboembolism (VTE), family history of idiopathic VTE and other known risk factors for VTE.
3) Ischaemic heart disease, severe hypertension or coagulation abnormalities.
4) Liver disease including disorders of hepatic excretion e.g. Dubin-Johnson or Rotor syndromes, infective hepatitis (until liver function returns to normal), known or suspected disorders of lipid metabolism, porphyria, liver adenoma or carcinoma, gallstones or jaundice with prior pill use.
5) Sickle cell anaemia.
6) Known or suspected carcinoma of the breast or estrogen dependent neoplasms.
7) Undiagnosed abnormal vaginal bleeding.
8) History during pregnancy of idiopathic jaundice, severe pruritus, chorea, pemphigoid gestationis or deterioration of otosclerosis.
9) Focal, severe or crescendo migraine or transient cerebral ischaemic attacks without headaches.
10) Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
The following information is principally based on studies in patients who used oral contraceptives with higher concentrations of estrogens and progestogens than those in common use today. The effect of long-term use of the oral contraceptives with lower concentrations of both estrogens and progestogens remains to be determined. The efficacy of any contraceptive method, except sterilisation, depends upon the reliability with which it is used. Correct and consistent use of such methods can result in lower failure rates.
Thrombo-embolism
An increased risk of thromboembolic disease (VTE) associated with the use of oral contraceptives is well established but is smaller than that associated with pregnancy,
which has been estimated at 60 cases per 100,000 pregnancies. Some epidemiological studies have reported a greater risk of VTE for women using combined oral contraceptives containing desogestrel or gestodene (the so-called ‘third generation’ pills) than for women using pills containing levonorgestrel or norethisterone (the so-called ‘second generation’ pills).
The spontaneous incidence of VTE in healthy, non-pregnant women (not taking any oral contraceptive) is about 5 cases per 100,000 per year. The incidence in users of second generation pills is about 15 per 100,000 women per year of use. The incidence in third generation pills is about 25 cases per 100,000 women per year of use; this excess incidence has not been satisfactorily explained by bias or confounding. The risk of venous thromboembolism is highest during the first year a combined oral contraceptive is taken. This increased risk applies to the first time ever combined oral contraceptive use is begun rather than each time a woman starts a new type of combined oral contraceptive. The level of all these risks of VTE increases with age and is likely to be further increased in women with other known risk factors for VTE such as obesity. The suitability of combined oral contraceptives for patients with any of these risk factors should be discussed with the patient before a final decision is taken.
The physician should be alert to the earliest manifestations of these disorders (thrombophlebitis, cerebrovascular disorders, pulmonary embolism and retinal thrombosis). Should any of these occur or be suspected, Loestrin should be discontinued immediately.
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes a day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
Hepatic tumours
Benign hepatic tumours have been associated with oral contraceptive usage. Malignant hepatic tumours have also been reported on rare occasions in long term users of oral contraceptives. A hepatic tumour should be considered in the differential diagnosis when upper abdominal pain, enlarged liver or signs of intra-abdominal haemorrhage occur.
Ovarian, endometrial, cervical and breast cancer
Numerous epidemiological studies have been reported on the risks of ovarian, endometrial, cervical and breast cancer in women using combined oral contraceptives. The evidence is clear that combined oral contraceptives offer substantial protection against both ovarian and endometrial cancer.
The most important risk factor for cervical cancer is persistent HPV infection. Some epidemiological studies have indicated that long-term use of COCs may further contribute to this increased risk but there continues to be controversy about the extent to which this finding is attributable to confounding effects, e.g., cervical screening and sexual behaviour including use of barrier contraceptives.
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using combined oral contraceptives (COCs). The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or, a combination of both. The additional breast cancers diagnosed in current users of COCs, or in women who have used COCs in the last ten years, are more likely to be localised to the breast than in those women who have never used COCs.
Breast cancer is rare among women under 40 years of age, whether or not they take COCs. Whilst this background risk increases with age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer (see bar chart).
The most important risk factor for breast cancer in COC users is the age women discontinue the COC; the older the age at stopping, the more breast cancers are diagnosed. Duration of use is less important and the excess risk gradually disappears during the course of the ten years after stopping COC use, such that by 10 years there appears to be no excess.
The possible increase in risk of breast cancer should be discussed with the user and weighed against the benefits of COCs taking into account the evidence that they offer substantial protection against the risk of developing certain other cancers (e.g. ovarian and endometrial cancer).
Estimated cumulative numbers of breast cancers per 10,000 women diagnosed in 5 years of use and up to 10 years after stopping COCs, compared with numbers of breast cancers diagnosed in 10,000 women who had never used COCs
□
Q Never took COCs Used COCs for 5 years
Number of breast cancers
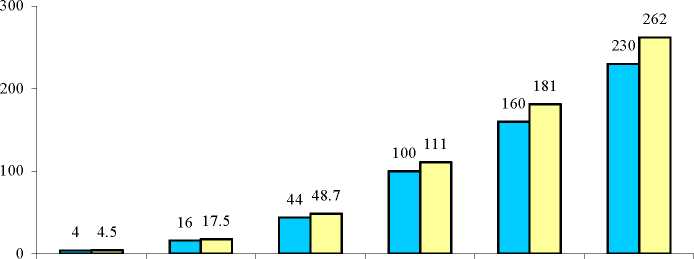
T°°k the KH at these ages: Under 20 20-24 25-29 30-34 35-39 40-44
Cancers found up to the age of: 30 35 40 45 50 55
Reasons for stopping Loestrin immediately
1) Occurrence of migraine in patients who have never previously suffered from it. Any unusually frequent or severe headaches.
2) Any kind of visual disturbance e.g. proptosis or diplopia and migraine.
3) Suspicion of thrombosis or infarction.
4) Combined oral contraceptives should be stopped at least six weeks before elective surgery and during and following prolonged immobilisation e.g. after accidents, etc.
5) Loestrin should be discontinued if the patient becomes jaundiced or has a significant rise in blood pressure.
6) Patients with a history of depression should be carefully observed and the drug discontinued if the depression recurs to a serious degree.
7) Since the safety of Loestrin in pregnancy has not been demonstrated, it is recommended that for any patient who has missed a period, the absence of pregnancy should be established before continuing the contraceptive regimen.
8) Clear exacerbation of conditions known to be capable of deteriorating during oral contraception or pregnancy.
Assessment of women prior to starting oral contraceptives (and at regular intervals
thereafter) should include a personal and family medical history of each woman.
Physical examination should be guided by this and by the contraindications (section 4.3) and warnings (section 4.4) for this product. The frequency and nature of these assessments should be based upon relevant guidelines and should be adapted to the individual woman, but should include measurement of blood pressure and, if judged appropriate by the clinician, breast, abdominal and pelvic examination including cervical cytology.
In case of undiagnosed, persistent or recurrent abnormal vaginal bleeding, appropriate diagnostic measures should be conducted to rule out malignancy. Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
Estrogen-progestogen preparations should be used with caution in patients with a history of hypertension and some women experience an increase in blood pressure following the administration of contraceptive steroids. Pregnancy should be excluded before starting treatment. Because these agents may cause some degree of fluid retention, patients with conditions which might be influenced by this such as epilepsy, migraine, asthma, and cardiac or renal dysfunction should be carefully monitored.
A decrease in glucose tolerance has been observed in a significant percentage of patients on oral contraceptives. The mechanism of this decrease is obscure. For this reason, pre-diabetic and diabetic patients should be carefully observed whilst receiving Loestrin.
Under the influence of estrogen-progestogen preparations, pre-existing uterine fibroleiomyomata may increase in size. Loestrin may mask the onset of the climacteric.
The following conditions also require careful consideration: multiple sclerosis, porphyria, tetany, disturbed liver function, gallstones, cardiovascular disease, renal disease, chloasma or any disease that is prone to worsen during pregnancy. The deterioration or first appearance of any of these conditions may indicate that the oral contraceptive should be stopped. Contact lens wearers who develop visual changes or changes to lens tolerance should be assessed by an optometrist.
Interference with laboratory tests
The following laboratory results may be altered by the use of oral contraceptives: hepatic function (increased sulpho-bromophthalein retention and other tests); thyroid function (increased thyroid binding globulin (TBG) leading to increased circulating total thyroid hormone as measured by protein-bound iodine (PBI), T4 by column or by radioimmunoassay. Free T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 concentration is unaltered); haematological tests (increased prothrombin and factors VII, VIII, IX and X, decreased antithrombin 3 and increased adrenaline induced platelet aggregation); measurement of pregnanediol excretion (reduced). Other binding proteins may be elevated in the serum, sex-binding globulins are increased, triglycerides may be increased and serum folate levels may be depressed. Therefore, if such tests are abnormal in a patient taking Loestrin, it is recommended that they be repeated after Loestrin has been withdrawn for two months. The pathologist should be advised of the administration of Loestrin when relevant specimens are submitted. Any influence of prolonged administration of Loestrin on pituitary, ovarian, adrenal, hepatic and uterine functions is unknown at present.
This product contains lactose, therefore patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
This product contains sucrose, therefore patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine.
4.5 Interactions with Other Medicaments and Other Forms of Interaction
Effects of other drugs on oral contraceptives
The effectiveness of combined oral contraceptives may be considerably reduced by interaction with drugs that induce hepatic enzyme activity e.g. carbamazepine, griseofulvin, phenytoin, phenobarbital, primidone and rifampicin.
Additional contraceptive precautions should be taken whilst taking enzyme inducing drugs and for at least seven days after stopping them. If these seven days run beyond the end of the packet, the new packet should be started immediately without a break. Rifampicin is such a potent inducer that even if the course lasts for less than 7 days, the additional contraceptive precautions should be continued for at least 4 weeks after stopping it.
The herbal remedy St. John’s Wort (Hypericumperforatum) should not be taken concomitantly with this medicine as this could potentially lead to a loss of contraceptive effect.
Ascorbic acid and paracetamol may increase plasma ethinylestradiol concentrations, possibly by inhibition of conjugation.
Administration of atorvastatin concomitantly with oral contraceptives containing ethinylestradiol and norethisterone acetate increased AUC values for norethisterone and ethinylestradiol by approximately 30% and 20% respectively.
Effects of oral contraceptives on other drugs:
Oral contraceptive combinations containing ethinylestradiol may inhibit the metabolism of other compounds. Increased plasma concentrations of ciclosporin, prednisolone and theophylline have been reported with concomitant administration of oral contraceptives. In addition, oral contraceptives may induce conjugation of other compounds. Decreased plasma concentrations of paracetamol have been noted when administered with oral contraceptives.
4.6 Pregnancy and lactation
Loestrin is not recommended for use during pregnancy, suspected pregnancy and in lactating mothers. Studies do not suggest a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when oral contraceptives are taken inadvertently during early pregnancy. The administration of oral contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy. Oral contraceptives should not be used during pregnancy to treat threatened or habitual abortion.
4.7 Effects on ability to drive and use machines
None known
4.8 Undesirable Effects
The following adverse effects which have been reported in patients receiving oral contraceptives are believed to be drug-related:
Nausea, vomiting, gastro-intestinal symptoms (such as abdominal cramps and bloating), breakthrough bleeding, spotting, change in menstrual flow, amenorrhoea during and after treatment, oedema, chloasma or melasma, breast changes (tenderness, enlargement and secretion), change in weight, cervical erosion and changes in cervical secretion, suppression of lactation when given immediately post-partum, cholestastic jaundice, migraine, rash (allergic), rise in blood pressure, depression, thrombo-embolic disorders, temporary infertility after discontinuation of treatment, reduced tolerance to carbohydrates, vaginal candidiasis, change in corneal curvature (steepening) and intolerance to contact lenses.
Although the following adverse effects have been reported in women taking oral contraceptives, an association has been neither confirmed nor refuted: prolonged amenorrhoea after discontinuing oral contraceptives, pre-menstrual like syndrome, headache, nervousness, dizziness, fatigue, cataract, backache, hirsutism, loss of scalp hair, erythema multiforme, erythema nodusum, haemorrhagic eruption, itching, changes in appetite, cystitis-like syndrome, vaginitis, porphyria, impaired renal function, haemolytic uraemic syndrome, Budd-Chiari syndrome, acne, changes in libido and colitis.
Menstrual changes
Breakthrough bleeding and spotting are sometimes encountered, especially during the first three months of use. Non-hormonal causes should be considered and adequate diagnostic measures taken to rule out malignancy or pregnancy in the event of breakthrough bleeding, as in the case of any abnormal vaginal bleeding. If pathology has been excluded, time or a change to another formulation may solve the problem.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
The usual effects in children are nausea and drowsiness. Slight vaginal bleeding occasionally occurs in girls. In view of the low toxicity following overdosage with oral contraceptives, it is suggested that treatment should be conservative.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Loestrin achieves its contraceptive effect primarily by inhibition of ovulation through gonadotrophin suppression. It is thought that other sites of action such as changes in cervical mucus and in the endometrium may contribute to the efficacy of combined oral contraceptives.
5.2 Pharmacokinetic Properties
Ethinylestradiol is rapidly and almost completely absorbed and peak serum levels are usually attained within an hour of oral administration. At this time, the majority of drug is already conjugated, largely as the sulfate. These conjugates have a primary serum half-life of approximately 7 hours and a terminal half-life of 48 hours and are excreted in urine and faeces.
Norethisterone acetate undergoes rapid absorption with peak serum concentrations occurring at one hour after oral administration. Less than 5% is cleared as unchanged norethisterone; glucuronide and sulfate conjugates are excreted in urine and faeces. The terminal half-life for norethisterone conjugates has been estimated at 70 hours (range: 42 - 84 hours).
5.3 Preclinical safety data
The results of the preclinical tests do not add anything of further significance to the prescriber.
6.1 List of excipients
Lactose,
Sucrose,
Maize starch,
Talc,
Spray-dried acacia Magnesium stearate Hypromellose 15 Carnauba wax Hydroxypropylcellulose Quinoline yellow (E104) Sunset yellow FCF (E110) Patent blue V (E131) Indigo carmine (E132) Brilliant blue FCF (E133) Titanium dioxide (E171)
6.2 Incompatibilities
None known
6.3 Shelf life
3 years.
6.4 Special precautions for storage
Do not store above 30°C. Store in the outer carton.
6.5 Nature and contents of container
Printed aluminium foil blister strip contained in a cardboard carton together with a product leaflet. Supplied in packs of 21 and 63 tablets.
6.6 Special precautions for disposal
No special instructions needed.
7 MARKETING AUTHORISATION HOLDER
Galen Limited
Seagoe Industrial Estate
Craigavon
BT63 5UA
UK.
8 MARKETING AUTHORISATION NUMBER(S)
PL 27827/0024.
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
03 April 1974/11 February 2009.
10 DATE OF REVISION OF THE TEXT
28/09/2015