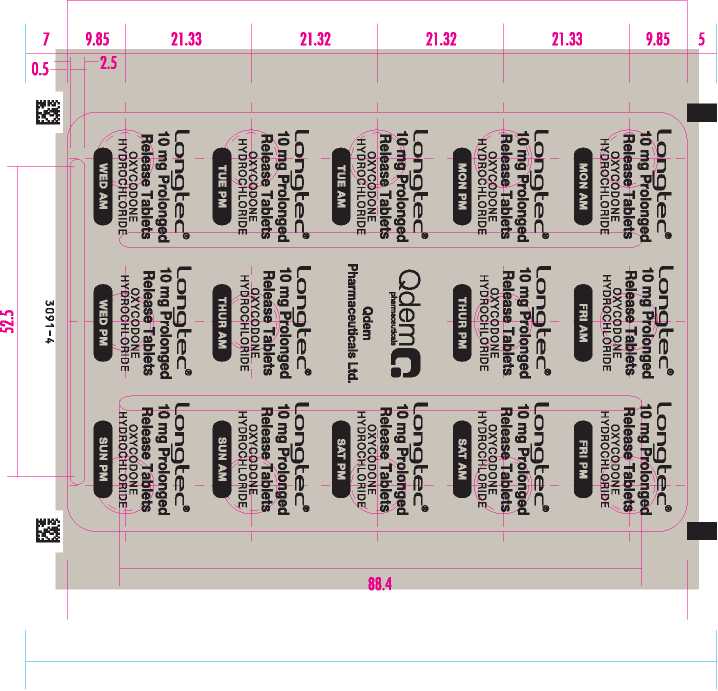
Longtec 10 Mg Prolonged Release Tablets
prolonged release taoieis oxycodone hydrochloride

take by mouth ovary 12 holes
LonqLc
prolonged release tablets oxycodone hydrochloride
|
E |
I- I I < LLI tr |
Each prolonged release tablet contains 19 mg of oxycodone as 10 mg oxycodone hydrochloride. | Also includes: Lactose monohydrate. Pleese reed the leoflet corefully before use. | |
|
LJ |
I |
< £2 |
1 Dosage: To be taken as directed by the |
|
E |
| physician. | ||
|
ra |
I |
i— |
Tablets must he swallowed whole |
|
| |
| and not broken, chewed or crushed | ||
|
L |
I |
LLI 00 5 |
. Keep out of the reach and sight of children. 1 Do not store above 25'C. |
|
>« |
>- |
| This medicine cen meke you feel sleepy. | |
|
u. |
I |
< |
Do not drive while teking this medicine until |
|
0 |
I |
| you know how it makes you feel. | |
|
r- |
< |
See the leaflet inside for more informetion. | |
|
I |
nz Q. |
1 Qdem Phermeceuticals Ltd. | |
|
.E |
>3 |
. Cambridge Science Park, Milton Road | |
|
cu |
I |
CL o_ |
Cambridge CB40AB, UK |
|
CO |
< |
| ® LONGTEC and QDEM are Registered Trade Marks. | |
|
-C |
I |
© 2012-2014 Qdem Pharmaceuticals Limited | |
|
TO |
I |
1 PL40431/0002 ,-, \nn 7 | POM | | |
53;
Ti -
10 mg
06
T
07 92
take by mouth every 12 hours
• # • •• e# •
• • • •
• • • • •• • e
Lonqb
prolonged release tablets oxycodone hydrochloride
56 tablets For oral use
f------1
take by mouth every 12 hours
Lonqbe
prolonged release tablets oxycodone hydrochloride
17
NAP6BR 74x32.5x110mm
• • •• • •
10 mg
QdemC
pharmaceuticals
56 tablets
10 mg
LONGTEC TABS 10MG CTN 56S UK 3086-5 VI.pdf 1 04/12/2015

r>
NAPP BLISTER DRAWING No. BSN-L-040 NAPP BLISTER LAYOUT DRAWING No. FSN-NAPP-04 V2

POCKET DETAILS 9.7
tn
e>i
e>i
105
REEL WIDTH = 117mm
II