Loramyc 50Mg Muco-Adhesive Buccal Tablets
Miconazole Lauriad 50 mg, muco-adhesive buccal tablet Package Leaflet
PACKAGE LEAFLET: INFORMATION FOR THE USER
LORAMYC 50 mg, muco-adhesive buccal tablet
Miconazole
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have further questions, ask your doctor or your pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them even if their symptoms are the same of yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What LORAMY C is and what it is used for.
2. Before you use LORAMYC.
3. How to use LORAMYC.
4. Possible side effects.
5. How to store LORAMYC.
6. Further information.
1. WHAT LORAMYC 50 mg, tablet IS AND WHAT IT IS USED FOR
This medicinal product is used for the treatment of fungal (yeast) infections of the mouth and the throat due to Candida in patients with reduced immune system.
2. BEFORE YOU USE LORAMYC 50 mg, tablet
Do not use LORAMYC 50 mg, tablet:
- If you are allergic (hypersensitive) to miconazole, other medicines of the azoles group or any of the other ingredients of LORAMYC,
- If you are allergic to milk or milk derivatives,
- If you suffer from liver failure (liver dysfunction)
- With oral anticoagulants (medicines that slow down blood clotting), hypoglycaemic sulfonamides (medicines used for the treatment of diabetes), cisapride (a medicine used for the treatment of gastro-oesophageal reflux), pimozide (a medicine used for the treatment of psychiatric disorders), and ergot alkaloids: ergotamine, dihydroergotamine (medicines used for the treatment of migraine) (see “Using other medicines”).
Take special care with LORAMYC 50 mg, tablet
- Avoid all situations that could interfere with adhesion of the tablet: do not touch or press where the tablet is placed, avoid chewing-gum;
- If brushing your teeth during the day, be careful not to touch the tablet during brushing and be careful when rinsing your mouth;
- If your mouth is dry, humidify your gum before applying the tablet.
- If LORAMYC is accidentally swallowed, it is recommended to drink a glass of water. If swallowed within the first 6 hours after application, it should be replaced only once.
- If the tablet falls off within the first 6 hours but is not swallowed, it should be replaced immediately.
Using other medicines with LORAMYC
LORAMYC must not be used if you are already using any of these medicines:
- oral anticoagulants (medicines that slow down blood clotting),
- cisapride (a medicine used for the treatment of gastro-oesophageal reflux),
- pimozide (a medicine used for the treatment of psychiatric disorders),
- hypoglycaemic sulfonamides (medicines used for the treatment of diabetes)
- ergot alkaloids: ergotamine, dihydroergotamine (medicines used for the treatment of migraine).
LORAMYC is not recommended if you are already using:
- halofantrine (a medicine used for the treatment of malaria).
LORAMYC should be avoided if possible if you are already using:
- phenytoine (a medicine used for the treatment of epilepsy).
Please tell your doctor or your pharmacist if you are using or have recently used any other medicines including medicines obtained without a prescription.
Using LORAMYC with food and drink
LORAMYC can be used with food and drinks.
Pregnancy:
It is preferable to avoid the use of this medicine during pregnancy.
If you discover that you are pregnant during the treatment, consult your doctor. Only your doctor can consider the necessity to maintain the treatment.
Ask your doctor or your pharmacist for advice before taking any medicine.
Breast-feeding:
It is not known if LORAMYC passes into breast milk. Ask you doctor for advice before using this medicinal product.
If your child is treated with a medicinal product which contains cisapride, you must not use this medicinal product during lactation.
Ask your doctor or your pharmacist for advice before using any medicine.
Driving and using machines:
LORAMYC is not expected to have any effects on the ability to drive or use machines.
Important information about some of the ingredients of LORAMYC 50 mg, tablet:
LORAMYC contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before using this medicine.
Do not use if you are allergic to milk or milk derivatives.
3. HOW TO USE LORAMYC 50 mg, tablet
DO NOT SWALLOW LORAMYC Instructions for proper use
LORAMYC must be applied to the upper gum, just above a front tooth.
- Before applying the tablet, locate the area on the upper gum, above the front tooth.
- Open the bottle and take out a tablet. Apply the tablet immediately after taking it out from the bottle. You will notice that the tablet has a rounded side and a flat side marked with “L”;
- Place the rounded side on the upper gum;
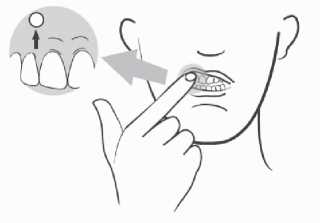
- Hold the tablet in place by applying a slight pressure with your finger on the outside of your upper lip for 30 seconds. This will allow the tablet to stick to your gum. Avoid touching the tablet with your tongue for several minutes thereafter;
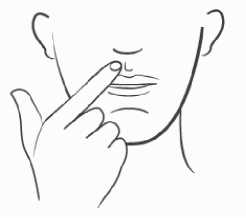
- Apply LORAMY C in the morning, preferably after brushing your teeth; do not suck, chew or swallow the tablet;
- If you accidentally swallow a tablet of LORAMY C, drink a glass of water.
- With each application, use each alternate sides of your gum.
- Reposition the tablet if it does not adhere properly
The shape of the tablet may change due to absorbing saliva in order to fit your gum shape.
Dosage
Always use LORAMYC exactly as your doctor has told you. You should check with your doctor or your pharmacist if you are not sure.
This medicine is only for adults.
The usual dose is one tablet of LORAMYC applied once a day.
Apply LORAMYC to the upper gum, in the morning, preferably after brushing your teeth.
If you feel that the effect of LORAMYC 50 mg tablet is too strong or too weak, consult your doctor or your pharmacist.
Duration of treatment
The usual duration of treatment is 7 to 14 days.
If you use or swallow more LORAMYC 50 mg tablet than you should:
In the event of overdose, you may experience nausea and vomiting. Consult your doctor or your pharmacist immediately.
If you forget to use LORAMYC 50 mg tablet:
Do not take a double dose to make up for a forgotten tablet.
If you stop using LORAMYC 50 mg tablet:
Do not stop using this medicine except on your doctor’s advice. Stopping the course early may lead to the failure of the treatment.
If you have any further questions on the use of this product, ask your doctor or your pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, LORAMYC 50 mg, tablet can cause side effects, although not everybody gets them:
Common side effects (greater than 1 in 100, less than 1 in 10 patients) are:
- Nausea, diarrhea, abdominal pain, vomiting, dry mouth, oral discomfort, gingival pain,
- Headache, change in taste,
- Itching, rash.
Uncommon side effects (greater than 1 in 1000, less than 1 in 100 patients) are:
- Tongue pain, gingival itching, mouth ulceration
- Application site irritation (gum), fatigue, pain,
- Infection of nose and throat,
- Appetite loss,
- Hot flush.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or your pharmacist.
5. HOW TO STORE LORAMYC 50 mg tablet
Keep out of reach and sight of children.
Store below 30°C.
Keep in the bottle tightly closed in order to protect from moisture.
Do not use LORAMYC after the expiry date printed on the bottle and on the box. The expiry date refers to the last day of that month.
6. FURTHER INFORMATION
What LORAMYC contains:
The active substance is Miconazole.
Each muco-adhesive buccal tablet contains 50 mg of miconazole.
The other ingredients are hypromellose 2208, milk protein concentrate, maize starch, lactose monohydrate, sodium laurilsulfate, magnesium stearate, talc.
What LORAMYC looks like and contents of the pack
This medicine is a muco-adhesive buccal tablet, white to slightly yellow with a rounded side and a flat side marked with “L”. Each box of LORAMYC contains one bottle of 14 tablets.
Marketing authorisation holder:
Onxeo
49 Boulevard du General Martial Valin
75015 Paris
France
Manufacturer:
Catalent Germany Schorndorf GmbH
SteinbeisstraBe 2
73614 Schorndorf
Postfach 1460
73603 Schorndorf
Germany
This medicine is authorised in the Member States of the EEA under the following names:
France, Germany, Italy, United Kingdom: LORAMYC This leaflet was last approved in
Onxeo Confidential 5/5