Lorazepam 1Mg/Ml Oral Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Lorazepam 1mg/ml Oral Solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of oral solution contains 1mg lorazepam.
Excipients with known effect:
Each ml of oral solution contains 20.21mg ethanol (alcohol).
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Oral Solution
A clear, colourless to pale yellow colour oral solution
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
FOR SHORT TERM (2-4 weeks only) USE
• Symptomatic relief of anxiety that is severe, disabling or subjecting the individual to unacceptable distress occurring alone or in association with insomnia or shortterm psychometric, organic or psychotic illness.
AS PREMEDICATION
• Before operative dentistry and general surgery NOT FOR USE
• Long term (i.e. longer than 4 weeks)
• For mild/moderate anxiety
Lorazepam Oral Solution is not recommended for use in children.
4.2 Posology and method of administration
Route of administration: oral Treatment to be given:
• Under close medical supervision
• At the lowest effective dose
• For the shortest possible duration (not exceeding 4 weeks)
Doses should be individualised
Extension of use should not take place without further clinical evaluation
Chronic use not recommended (little is known of the long term safety and efficacy; potential for dependence) (see section 4.4)
When treatment is started the patient should be informed that
• Treatment will be of limited duration
• The dosage will be progressively decreased
• There is a possibility of rebound phenomena Dosage:
Adults:
• Anxiety: 1 - 4ml (1 - 4mg) daily in divided doses.
• Insomnia: 1 - 2ml (1 - 2mg) before retiring
• Premedication before operative dentistry or general surgery: 2 - 3ml (2 - 3mg) the night before operation 2 - 4ml (2 - 4mg) one to two hours before the procedure
Elderly:
The elderly may respond to lower doses (half normal adult dose or less)
Patients with Renal or Hepatic impairment:
Lower doses may be sufficient in these patients (see section 4.4). Use in patients with severe hepatic insufficiency is contraindicated (see section 4.6)
Method of administration
The required dose should be drawn from the container into the graduated syringe provided using the syringe adaptor (see detailed instructions below). The syringe should be held in the mouth of the patient, and the contents of the syringe should then be ejected into the mouth and swallowed.
a) Open the bottle: press the cap and turn it anticlockwise (figure 1).
b) Separate the adaptor from the syringe (figure 2). Insert the adaptor into the bottle neck (figure 3). Ensure it is properly fixed. Take the syringe and put it in the adaptor opening (figure 4).
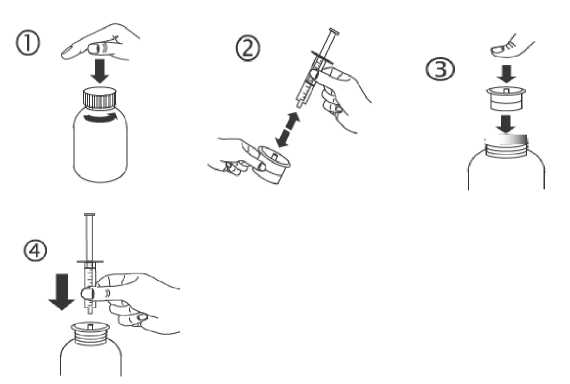
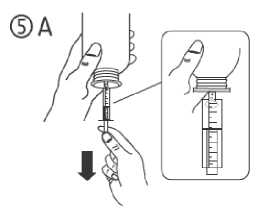
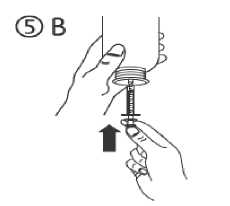
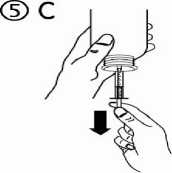
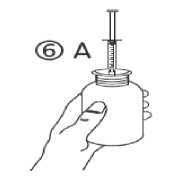
c) Turn the bottle upside down. Fill the syringe with a small amount of solution by pulling the piston down (figure 5A), then push the piston upwards in order to remove any possible bubble (figure 5B). Pull the piston down to the graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (figure 5C).
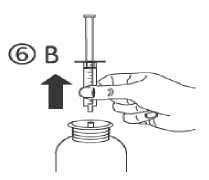
d) Turn the bottle the right way up (figure 6A). Remove the syringe from the adaptor (figure 6B).
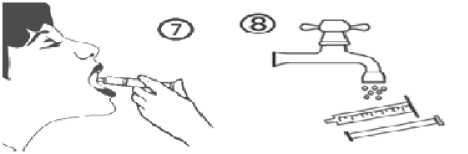
e) Empty the contents of the syringe into the patient’s mouth by pushing the piston to the bottom of the syringe (figure 7). Close the bottle with the plastic screw cap. Wash the syringe with water (figure 8).
4.3 Contraindications
• Hypersensitivity to benzodiazepines or to any of the other ingredients
• Acute pulmonary insufficiency: respiratory depression; sleep apnoea (risk of further respiratory depression)
• Obsessional states (inadequate evidence of safety and efficacy)
• Severe hepatic insufficiency (may precipitate encephalopathy)
• Planning a pregnancy (see section 4.6)
• Pregnancy (unless there are compelling reasons-see section 4.6)
• Myasthenia gravis;
Benzodiazepines should not be used alone in depression or anxiety with depression (may precipitate suicide)
4.4 Special warnings and precautions for use
• Patients should be advised that since their tolerance for alcohol and other CNS depressants will be diminished in the presence of Lorazepam, these substances should either be avoided or taken in reduced dosage.
• Lorazepam is not intended for the primary treatment of psychotic illness or depressive disorders, and should not be used alone to treat depressed patients. The use of benzodiazepines may have a disinhibiting effect and may release suicidal tendencies in depressed patients. Therefore, large quantities of Lorazepam should not be prescribed to these patients.
• Pre-existing depression may emerge during benzodiazepine use.
• The use of benzodiazepines may lead to physical and psychological dependence. The risk of dependence on Lorazepam is low when used at the recommended dose and duration, but increases with higher doses and longer-term use. The risk of dependence is further increased in patients with a history of alcoholism or drug abuse, or in patients with significant personality disorders. Therefore, use in individuals with a history of alcoholism or drug abuse should be avoided.
• Dependence may lead to withdrawal symptoms; especially if treatment is discontinued abruptly (see section 4.8). Therefore, the drug should always be discontinued gradually.
• It may be useful to inform the patient that treatment will be of limited duration and that it will be discontinued gradually. The patient should also be made aware of the possibility of "rebound" phenomena to minimise anxiety should they occur.
• Abuse of benzodiazepines has been reported.
• Some loss of efficacy to the hypnotic effects of short-acting benzodiazepines may develop after repeated use for a few weeks.
• Anxiety or insomnia may be a symptom of several other disorders. The possibility should be considered that the complaint may be related to an underlying physical or psychiatric disorder for which there is more specific treatment.
• Caution should be used in the treatment of patients with acute narrow-angle glaucoma.
• Patients with impaired renal or hepatic function should be monitored frequently and have their dosage adjusted carefully according to patient response. Lower
doses may be sufficient in these patients. The same precautions apply to elderly or debilitated patients and patients with chronic respiratory insufficiency.
• As with all CNS-depressants, the use of benzodiazepines may precipitate encephalopathy in patients with severe hepatic insufficiency. Therefore, use in these patients is contraindicated.
• Some patients taking benzodiazepines have developed a blood dyscrasia, and some have had elevations in liver enzymes. Periodic haematologic and liver-function assessments are recommended where repeated courses of treatment are considered clinically necessary.
• Transient anterograde amnesia or memory impairment has been reported in association with the use of benzodiazepines. This effect may be advantageous when Lorazepam is used as a premedicant. However, if Lorazepam is used for insomnia due to anxiety, patients should ensure that they will be able to have a period of uninterrupted sleep which is sufficient to allow dissipation of drug effect (e.g., 7-8 hours).
• Paradoxical reactions have been occasionally reported during benzodiazepine use. Such reactions may be more likely to occur in the elderly. Should these occur, use of the drug should be discontinued (see Undesirable effects).
• Although hypotension has occurred only rarely, benzodiazepines should be administered with caution to patients in whom a drop in blood pressure might lead to cardiovascular or cerebrovascular complications. This is particularly important in elderly patients.
Excipient Warnings:
This medicinal product contains small amount of ethanol (alcohol), less than 100mg
per ml. The amount of ethanol 96% in each ml is 20.21mg.
4.5 Interaction with other medicinal products and other forms of interaction
Not recommended
> Alcohol: Lorazepam should not be used together with alcohol (enhanced sedative effects; impaired ability to drive/operate machinery)
> Sodium oxybate: Avoid concomitant use (enhanced effects of sodium oxybate)
> HIV-protease inhibitors: Avoid concomitant use (increased risk of prolonged sedation - see below for zidovudine.
Take into account
> Centrally acting drugs: Enhancement of the central depressive effect may occur if lorazepam is combined with drugs such as neuroleptics, antipsychotics, tranquillisers, antidepressants, hypnotics, analgesics, anaesthetics, barbiturates and sedative antihistamines. The elderly may require special supervision.
> Anti-epileptic drugs:
• Pharmacokinetic studies on potential interactions between
benzodiazepines and antiepileptic drugs have produced conflicting results. Depression and elevation of drug levels, as well as no change have been reported.
• Phenobarbital taken concomitantly may result in an additive CNS effect. Special care should be taken in adjusting the dose in the initial stages of treatment.
• Side effects may be more evident with hydantoins or barbiturates
• Valproate may inhibit the glucuronidation of lorazepam (increased serum levels: increased risk of drowsiness)
> Narcotic analgesics: Enhancement of the euphoria may lead to increased psychological dependence
> Clozapine: Reports of marked sedation, excessive salivation, hypotension, ataxia, delirium and respiratory arrest when given concurrently with lorazepam.
> Other drugs enhancing the sedative effect of diazepam: Cisapride, lofexidine, nabilone, disulfiram and the muscle relaxants - baclofen and tizanidine
> Compounds that affect hepatic enzymes (particularly cyctochrome P450)
• Inhibitors (e.g. cimetidine, isoniazid; erythyromycin; omeprazole; esomeprazole) reduce clearance and may potentiate the action of benzodiazepines. Itraconazole, ketoconazole and to a lesser extent fluconazole and voriconazole are potent inhibitors of the cytochrome P450 isoenzyme CYP3A4 and may increase plasma levels of benzodiazepines. The effects of benzodiazepines may be increased and prolonged by concomitant use. A dose reduction of the benzodiazepine may be required.
• Inducers (e.g. rifampicin) may increase clearance of benzodiazepines
> Antihypertensives, vasodilators and diuretics:
• Enhanced hypotensive effect with ACE-inhibitors, alpha-blockers, angiotensin-II receptor antagonists, calcium channel blockers, adrenergic neurone blockers, betablockers, moxonidine, nitrates, hydralazine, minoxidil, sodium nitroprusside and diuretics
• Enhanced sedative effect with alpha-blockers or moxonidine.
> Dopaminergics: Possible antagonism of the effect of levodopa
> Antacids: Concurrent use may delay absorption of lorazepam
> Zidovudine: Increased zidovudine clearance by lorazepam
> Oestrogen-containing contraceptives: Possible inhibition of hepatic metabolism of lorazepam
> Theophylline/aminophylline: Increases metabolism of lorazepam which possibly reduces the effect
> Caffeine: Concurrent use may result in reduced sedative and anxiolytic effects of lorazepam.
> Grapefruit juice: Inhibition of CYP3A4 may increase the plasma concentration of lorazepam (possible increased sedation and amnesia).This interaction may be of little significance in healthy individuals, but it is not clear if other factors such as old age or liver cirrhosis increases the risk of adverse events with concurrent use.
4.6 Fertility, pregnancy and lactation
Pregnancy: Benzodiazepines should not be used during pregnancy, especially during the first and last trimesters. Benzodiazepines may cause foetal damage when administered to pregnant women.
If the drug is prescribed to a woman of childbearing potential, she should be warned to contact her physician about stopping the drug if she intends to become, or suspects that she is, pregnant.
There is a possibility that infants born to mothers who take benzodiazepines chronically during the later stages of pregnancy may develop physical dependence. Infants of mothers who ingested benzodiazepines for several weeks or more preceding delivery have been reported to have withdrawal symptoms during the postnatal period. Symptoms such as hypoactivity, hypotonia, hypothermia, respiratory depression, apnoea, feeding problems, and impaired metabolic response to cold stress have been reported in neonates born of mothers who have received benzodiazepines during the late phase of pregnancy or at delivery.
Lactation: Lorazepam is excreted in small amounts in breast milk. Mothers who are breast-feeding should not take benzodiazepines. Sedation and inability to suckle have occurred in neonates of lactating mothers taking benzodiazepines.
4.7 Effects on ability to drive and use machines
Patients should be advised that sedation, amnesia, impaired concentration, dizziness, blurred vision and impaired muscular function may occur and that, if affected, they should not drive or to use machines, or take part in other activities where this would put themselves or others at risk. If insufficient sleep duration occurs, the likelihood of impaired alertness may be increased. Concurrent medication may increase these effects (see section 4.5).
This medicine can impair cognitive function and can affect a patient's ability to drive safely. This class of medicine is in the list of drugs included in regulations under 5a of the Road Traffic Act 1988. When prescribing this medicine, patients should be told:
• The medicine is likely to affect your ability to drive
• Do not drive until you know how the medicine affects you
• It is an offence to drive while under the influence of this medicine However, you would not be committing an offence (called 'statutory defence') if:
• The medicine has been prescribed to treat a medical or dental problem and
• You have taken it according to the instructions given by the prescriber and in the information provided with the medicine and
• It was not affecting your ability to drive safely.
4.8 Undesirable effects
Adverse reactions, when they occur, are usually observed at the beginning of therapy and generally decrease in severity or disappear with continued use or upon decreasing the dose.
Most frequently reported adverse reactions associated with benzodiazepines include daytime drowsiness, dizziness, muscle weakness, and ataxia.
Adverse reactions are listed by frequency:
Very common (>1/10); Common (>1/100 to < 1/10); Uncommon (>1/1,000 to < 1/100); Rare (>1/10,000 to <1/1,000); Very rare (<1/10,000), Not known (cannot be estimated from the available data).
|
System Organ Class |
Adverse Drug Reactions | |||||
|
Frequency Category | ||||||
|
Very Common (>1/10) |
Common (>1/100 to <1/10) |
Uncommon (>1/1000 to <1/100) |
Rare (>1/10000 to <1/1000) |
Very Rare (<1/10,000) |
Not Known | |
|
Blood and lymphatic System Disorders |
Thrombocytope nia, leucopenia, agranulocytosis, pancytopenia | |||||
|
Immune System Disorders |
Hyper sensitivity including anaphylaxis/ anaphylactoid reactions | |||||
|
Endo-crine Disorders |
Inappropriate antidiuretic hormone secretion, hyponatraemia | |||||
|
Psychia-tric Disorders |
Confusion, depression and unmasking of depression, numbed emotions, disinhibition, euphoria, appetite changes, sleep disturbance, change in libido, decreased orgasm. |
Depen dence, Suicidal ideation/ attempt | ||||
|
Paradoxical reactions such as restlessness, agitation, irritability, aggressiveness, delusion, rage, insomnia, nightmares, hallucinations, psychoses, sexual arousal, and inappropriate behaviour have been occasionally reported during use. | ||||||
|
System Organ Class |
Adverse Drug Reactions | |||||
|
Frequency Category | ||||||
|
Very Common (>1/10) |
Common (>1/100 to <1/10) |
Uncommon (>1/1000 to <1/100) |
Rare (>1/10000 to <1/1000) |
Very Rare (<1/10,000) |
Not Known | |
|
Nervous System Disorders |
Daytime drowsiness, sedation |
Dizziness, ataxia |
Headache, reduced alertness, dysarthria/ slurred speech, transient anterograde amnesia or memory impairment. |
Tremor, extrapyramidal reactions, Coma (see section 4.9) | ||
|
Eye Disorders |
Visual disturbances (diplopia, blurred vision) | |||||
|
Vascular Disorders |
Hypotension (see section 4.4) | |||||
|
Respira-tory, thoracic and mediastinal Disorders |
Apnoea, worsening of sleep apnoea, worsening of obstructive pulmonary disease. Respiratory depression (see section 4.9). | |||||
|
Gastro intestinal Disorders |
Nausea, constipation, salivation changes | |||||
|
Hepato-biliary Disorders |
Abnormal liver function test values (increases in bilirubin, transaminases, alkaline phosphatise), jaundice | |||||
|
Skin and Sub-cutaneous tissue disorders |
Rash, allergic dermatitis | |||||
|
System Organ Class |
Adverse Drug Reactions | |||||
|
Frequency Category | ||||||
|
Very Common (>1/10) |
Common (>1/100 to <1/10) |
Uncommon (>1/1000 to <1/100) |
Rare (>1/10000 to <1/1000) |
Very Rare (<1/10,000) |
Not Known | |
|
Musculo skeletal Disorders |
Muscle weakness | |||||
|
Repro-ductive System and Breast Disorders |
Impotence | |||||
|
General Disorders |
Asthenia, fatigue |
Hypothermia | ||||
Drug withdrawal symptoms (see section 4.4)
Symptoms reported following discontinuation of benzodiazepines include headaches, muscle pain, anxiety, tension, depression, insomnia, restlessness, confusion, irritability, sweating, and the occurrence of “rebound” phenomena whereby the symptoms that led to treatment with benzodiazepines recur in an enhanced form. These symptoms may be difficult to distinguish from the original symptoms for which the drug was prescribed.
In severe cases the following symptoms may occur: derealisation; depersonalisation; hyperacusis; tinnitus; numbness and tingling of the extremities; hypersensitivity to light, noise, and physical contact; involuntary movements; hyper-reflexia, tremor, nausea, vomiting; diarrhoea, abdominal cramps, loss of appetite, agitation, palpitations, tachycardia, panic attacks, vertigo, short-term memory loss, hallucinations/delirium; catatonia; hyperthermia, convulsions. Convulsions may be more common in patients with pre-existing seizure disorders or who are taking other drugs that lower the convulsive threshold such as antidepressants.
Reporting of suspected adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
4.9 Overdose
In the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken.
Overdosage of benzodiazepines is usually manifested by degrees of central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, mental confusion, and lethargy. In more serious cases, and especially when other CNS-depressant drugs or alcohol are ingested, symptoms may include ataxia, hypotension, hypotonia, respiratory depression, coma, and very rarely, death.
If ingestion was recent, induced vomiting and/or gastric lavage should be undertaken followed by general supportive care, monitoring of vital signs and close observation of the patient. If there is no advantage in emptying the stomach, activated charcoal may be effective in reducing absorption. Hypotension, though unlikely, may be controlled with noradrenaline. Lorazepam is poorly dialysable.
The benzodiazepine antagonist, flumazenil may be useful in hospitalised patients for the management of benzodiazepine overdosage. Flumazenil product information should be consulted prior to use.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Lorazepam is a benzodiazepine with anxiolytic, sedative and hypnotic properties. ATC code: N05BA06
5.2 Pharmacokinetic properties
Lorazepam is almost completely absorbed from the gastrointestinal tract and peak serum levels are reached in 2 hours. It is metabolised by a simple one-step process to a pharmacologically inert glucuronide. There are no major active metabolites. The elimination half-life is about 12 hours and there is minimal risk of excessive accumulation.
5.3 Preclinical safety data
Oesophageal dilation occurred in rats treated with lorazepam for more than one year at 6mg/kg/day.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Ethanol (96%)
Medium chain triglycerides
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
This product is incompatible with polystyrene.
6.3 Shelf life
24 months
60ml: Discard 30 days after first opening.
150ml: Discard 90 days after first opening.
6.4 Special precautions for storage
Store and transport refrigerated (2°C - 8°C).
Keep the bottle in the outer carton in order to protect from light.
For storage conditions after first opening of the medicinal product, see section 6.3
6.5 Nature and contents of container
Bottle: Type III Amber glass
Closure: Tamper-evident, child-resistant plastic cap with expanded polyethylene liner
Dosing Device: 1ml oral syringe with 0.01ml graduations and a LDPE syringe adaptor
Pack size: 60ml and 150ml.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Syri Limited, t/a Thame Laboratories Unit 4, Bradfield Road,
Ruislip, Middlesex,
HA4 0NU, UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 39307/0054
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
29/11/2016
10 DATE OF REVISION OF THE TEXT
29/11/2016