Lubion 25 Mg Powder For Solution For Injection
Package leaflet: Information for the user Lubion 25 mg powder for solution for injection
Progesterone
Patient self-administration: Subcutaneous injection only Healthcare administration: By s.c and i.m injection
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
- Lubion 25mg powder for solution for injection will be known as Lubion throughout this leaflet.
What is in this leaflet:
1. What Lubion is and what it is used for
2. What you need to know before you use Lubion
3. How to use Lubion
4. Possible side effects
5. How to store Lubion
6. Contents of the pack and other information
1. What Lubion is and what is it used for
Lubion contains the active ingredient Progesterone. Progesterone is a naturally occurring female sex hormone. The medicine works on the lining of the womb and helps you to become pregnant and to stay pregnant.
Lubion is for women who need extra progesterone while undergoing treatment in an Assisted Reproductive Technology (ART) programme who are unable to use or tolerate vaginal preparations.
2. What you need to know before you use Lubion Do not use Lubion
• If you are allergic (hypersensitive) to progesterone or any of the other ingredients of Lubion which are listed in section 6
• If you are suffering from vaginal bleeding (other than normal periods) that has not been evaluated by your doctor
• If you have a miscarriage and your doctor suspects some tissue is still in the womb
• If you have had a pregnancy outside of the womb (ectopic pregnancy)
• If you currently have or have had severe liver problems
• If you have known or suspected breast cancer or cancer of the reproductive tract
• If you have or have had blood clots in the legs, lungs, eyes or elsewhere in the body
• If you have porphyria disorders (a group of inherited or acquired disorders of certain enzymes)
• If during pregnancy you have suffered from jaundice (yellowing of eyes and skin due to liver problems) severe itching and/or blisters on the skin
• If you are under 18 years of age.
Take special care with Lubion
If you experience any of the following during treatment tell your doctor immediately as your treatment may need to be stopped. Also tell your doctor immediately if you experience these a few days after the last dosage.
• Heart attack (pains in the chest, or back pain and/or deep aching and throbbing in one or both arms a sudden shortness of breath, sweating, dizziness, lightheadedness, naseau, palpitations)
• Stroke (severe headache or vomiting, dizziness, faintness, or changes in vision or speech, weakness or numbness of an arm or leg.)
• Blood clots in the eyes or anywhere in the body (pain in your eyes or pain and swelling in your ankles, feet and hands)
• Worsening of depression
• Severe headaches, changes in vision.
Before treatment with Lubion
Tell your doctor if you have had or have any of the following before using Lubion as you will be monitored during treatment:
• Liver problems (mild or moderate)
• Epilepsy
• Migraine
• Asthma
• Heart or kidney problems
• Diabetes
• Depression
Other medicines and Lubion
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription and herbal medicines.
• Carbamazepine (used to treat seizures/fits)
• Rifampin (an antibiotic)
• Griseofulvin (an antifungal medicine)
• Phenytoin and Phenobarbital (used to treat epilepsy)
• St. John’s Wort-containing herbal products.
• Ciclosporine (a medicine for some types of inflammation and after organ transplants)
• Diabetic medicines
• Ketoconazole (an antifungal medicines)
Do not administer Lubion at the same time as any other injectable medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
• Lubion can be used during the first three months of pregnancy.
• This medicine should not be used during breast-feeding.
Driving and using machines
Do not drive or use any tools or machines if you feel drowsy and/or dizzy whilst taking Lubion.
How to use Lubion
3.
Always use Lubion exactly as your doctor has told you. Remember, Lubion should only be used under the supervision of a doctor experienced in treating fertility problems.
How much Lubion should you use and for how long?
The usual dose is a once daily injections of 25 mg (the contents of one vial and 1ml of solvent). The duration of treatment is usually until 12 weeks of confirmed pregnancy (i.e. 10 weeks of treatment).
How Lubion should be given
Lubion can be given either under your skin (by subcutaneous injection) for doses of 25mg or into a muscle (by the intramuscular route) for doses of 25 mg.
You will be able to administer 25 mg of Lubion subcutaneously after suitable advice and training by your doctor or healthcare professional.
Before you inject Lubion you will receive the following training and advice:
• Practice giving subcutaneous injections
• Where to inject your medicine
• How to prepare your solution for injection
• How to administer your medicine.
Please read the instructions below on preparing and administering Lubion.
The steps for self administration are:
A Preparing your injection
B Checking pack
C Withdrawal of solvent (water for injection)
D Mixing the water for injection with the powder E Filling the syringe
F Changing the injecting needle
G Removing air bubbles
H Injection by subcutaneous administration I Disposal of used items.
These steps are explained in full below.
IMPORTANT: each vial should only be used once.
The solution should be used immediately after the powder has dissolved. It should not be stored.
A. Preparing your injection
The Lubion powder must be dissolved before it is injected. It is important to keep everything as clean as possible, so start by washing your hands thoroughly and drying on a clean towel. Choose a clean area to prepare your medicine:
• One vial containing Lubion powder
The following items are not supplied with your medicine. Your doctor or pharmacist will supply these items.
• One syringe
• One large needle (typically 21G green needle to mix the solution with Lubion powder and for intramuscular administration)
• One small fine needle (typically 27G grey needle; for subcutaneous injection)
• Water for injection (a solvent to add to the Lubion powder)
• Two alcohol wipes
• A sharps container (for safe disposal of needles, vials etc.).
B. Checking pack
• The Lubion vial, syringe and needles all have protective covers.
• Check that all the covers are on firmly and if they are not on properly or are damaged, do not use them
• Make sure that the expiry date is still valid on the vial of Lubion and the water for injection. Do not use the products if outside the expiry date.
C. Withdrawal of solvent (water for injection)
• Open the water for injection as described in the instruction leaflet provided with the product or as instructed by your doctor
• Unpack the syringe, hold the syringe
• Take the packaging off the large 21G green needle, but keep the needle cover on
• Attach the needle to the syringe, then remove the needle cover
• With the syringe in one hand pick up the water for injection and withdraw 1ml of solvent as described in the manufacturer’s instruction leaflet or by your doctor
• Carefully set the syringe down taking care not to touch the needle
D. Mixing the water for injection with the powder
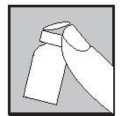
Remove the plastic cap from the top of a Lubion vial by gently pushing it upwards.
Wipe the rubber top with an alcohol wipe and allow to dry
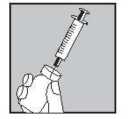
Push the large 21G green needle through the rubber middle of the Lubion vial top
Press the plunger down firmly to squirt all the solution onto the powder
Remove syringe with needle, place down carefully taking care not to touch the needle
When the powder is well soaked in the solvent, shake the vial vigorously to help the powder dissolve.
E. Filling the syringe
• Ensure the powder has dissolved (it may take up to 1 minute)
• The solution must be clear and colourless
• If the solution is cloudy or does not dissolve completely, do not use it and start again with another vial of Lubion
• Never use tap water or any liquid other than that given to you by your doctor or pharmacist
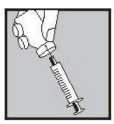
• Ensure that the plunger is at the bottom of the syringe then slowly push the large 21G green needle back through the rubber middle of the Lubion vial top.
• With the needle still inserted, turn the vial upside down The needle should support the vial unaided
• Make sure the needle tip is underneath the level of the liquid
• Gently pull the plunger to draw all the mixture into the syringe
• Pull the needle out of the vial.
F. Changing the injecting needle
This step is only required if you are doing a subcutaneous administration; if your doctor is performing an intramuscular injection they will move on to set the dose and administer the injection.
• Put the cap on the large 21G green needle and then gently take the large needle off the syringe
• Put this and the water for injection container into the sharps container you have been given
• Remove the smaller 27G grey injecting needle, from its package, keeping the needle cover on
• Attach the small 27G grey needle onto the syringe then remove the needle cover.
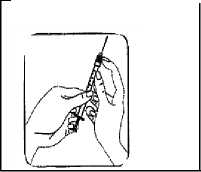
Holding the syringe straight up with the small 27G grey injecting needle pointing to the ceiling, draw back slightly on the plunger and tap the syringe so that any air bubbles rise to the top.
Slowly press the plunger until all the air is out of the syringe and at least one drop of solution comes out of the tip of the small 27G grey needle.
For all intramuscular injections, your doctor or another healthcare professional will perform the injection as described below.
H. Injection by subcutaneous administration
• Your doctor or healthcare professional will have already shown you where to inject Lubion (e.g. tummy or front of thigh)
• Open the alcohol wipe and carefully clean the area of skin to be injected, and allow it to dry
• Hold the syringe in one hand. Use the other hand to gently pinch the skin in the injection site area between your thumb and index finger
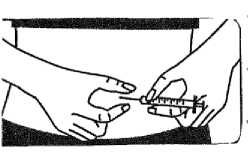
• Using a dart -like motion insert the fine grade small 27G grey needle into the skin so the skin and needle form a right angle.
• Insert the small 27G grey needle all the way into the skin. Do not inject directly into a vein
• Inject the solution by pushing gently on the plunger in a slow and steady motion until all the solution is injected beneath the skin. Inject all of the prescribed solution
• Release the skin and pull the needle straight out
• Wipe the skin at the injection site with an alcohol swab using a circular motion.
I. Disposal of used items:
• Once you have finished your injection, put all needles, empty vials and syringes into a sharps container.
• Any unused solution must be thrown away.
Intramuscular administration given by a doctor or healthcare professional
The Lubion injection will be given into the side of the thigh or bottom. Your doctor or healthcare professional will clean the area of skin to be injected using an alcohol wipe, and allow it to dry. Using a dart-like motion they will insert the large needle into the muscle. They will inject the solution by pushing gently on the plunger in a slow and steady motion until all the solution is injected into the muscle. They will pull the needle straight out and wipe the skin at the injection site with an alcohol wipe.
If you use more Lubion than you should
Tell your doctor or pharmacist. The symptoms of an overdose include drowsiness.
If you forget to use Lubion
Take the dose as soon as you remember and then carry on as before. Do not take a double dose to make up for a forgotten dose. Tell your doctor what you have done.
If you stop using Lubion
Do not stop using Lubion without speaking to your doctor or pharmacist first. Abrupt discontinuation of Lubion may cause increased anxiety, moodiness, and increase your risk of having seizures (fits).
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Lubion can cause side effects, although not everybody gets them.
Very common side effects (affecting up to 1 in 10 of every patient treated):
• Pain, redness, itching, irritation or swelling at the injection site
• Uterine spasm
• Vaginal bleeding.
Common side effects (affect between 1 in 10 and 1 in 100 of every patient treated):
• Overstimulation of the ovaries (symptoms include lower stomach pain, feeling thirsty and sick, and sometimes being sick, passing reduced quantities of concentrated urine and weight gain)
• Headache
• Bloated stomach
• Stomach pain
• Constipation
• Being sick and feeling sick
• Breast tenderness and/or pain
• Vaginal discharge
• A tingling or uneasy irritation or itching of the skin of the vagina and the surrounding area
• Hardening of area around injection site
• Bruising around injection site
• Fatigue (excessive tiredness, exhaustion, lethargy).
Uncommon side effects (affecting between 1 in 100 and 1in 1000 of every patient treated):
• Changes in mood
• Dizziness
• Insomnia
• Stomach and intestinal disturbance (including stomach discomfort and/or tenderness, wind, painful spasms and retching)
• Skin rashes (including red warm skin, or raised, itchy bumps or wheals or dry, cracked or blistered or swollen skin)
• Breast swelling and/or enlargement
• Feeling hot
• General feeling of discomfort or “feeling out of sorts”
• Pain.
The following disorders, although not reported by patients in clinical studies using Lubion, have been described with other progestins: depression, jaundice, insomnia, premenstrual like syndrome and menstrual disturbances, urticaria, acne, hirsutism, alopecia, weight gain and anaphylactoid reactions.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. How to store Lubion
Keep out of the sight and reach of children.
Store below 25°C.
Do not refrigerate or freeze.
Store in the original package in order to protect from light.
The medicinal product must be used immediately after first opening and reconstitution. Any remaining solution must be discarded.
Do not use Lubion after the expiry date which is stated on the label after Exp: The expiry date refers to the last day of that month.
Do not use Lubion if you notice particles in the solution or if the solution is not clear.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Lubion contains
The active substance is Progesterone.
One vial contains 25 mg of Progesterone.
After reconstitution with 1 ml water for injection, the reconstituted solution (1.119 ml) contains 25 mg progesterone.
The other ingredient is Hydroxypropylbetadex What Lubion looks like and contents of the pack
Lubion is a white powder for solution for injection supplied in a colourless glass vial. Each pack contains 1, 7 or 14 vials.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
IBSA Farmaceutici Italia Srl Via Martiri di Cefalonia 2 26900 Lodi (Italy)
Batch release in UK
Pharmasure Limited 4-6 Colonial Business Park Colonial Way Watford WD24 4PR
This leaflet was last approved in 04/2015.
If this leaflet is difficult to see or read or you would like it in a different format, please contact Pharmasure Limited, 4-6 Colonial Business Park, Colonial Way, Watford, WD24 4PR (Tel: +44(0) 1923 233466, email: info@pharmasure.co.uk)
8