Lutrate Depot 22.5 Mg Powder And Solvent For Prolonged-Release Suspension For Injection
Out of date information, search anotherPackage leaflet: Information for the user
Lutrate Depot 22.5 mg powder and solvent for prolonged-release suspension for
injection
leuprorelin acetate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Lutrate Depot is and what it is used for
2. What you need to know before you use Lutrate Depot
3. How to use Lutrate Depot
4. Possible side effects
5. How to store Lutrate Depot
6. Contents of the pack and other information
1. What Lutrate Depot is and what it is used for
Lutrate Depot is a vial containing a white powder, which is made into a suspension for injection into a muscle. Lutrate Depot contains the active ingredient leuprorelin (also called leuprolide), which belongs to a group of medicines called luteinizing hormone releasing hormone (LHRH) agonists (medicines that reduce testosterone - a sex hormone).
Your doctor has prescribed Lutrate Depot for palliative treatment of advanced prostate cancer.
Do not use Lutrate Depot:
- if you are allergic (hypersensitive) to LHRH, LHRH agonists or any of the other ingredients of this medicine (listed in section 6). An allergic reaction may include rash, itching, difficulty of breathing or swelling of the face, lips, throat or tongue.
- if you have had an orchiectomy (removal of the testicles).
- if you are female or a child.
- Lutrate Depot must not be used alone for the treatment of prostate cancer when the spinal cord is compressed or the cancer has spread to the spine.
Warnings and precautions
• Talk to your doctor or pharmacist before you are given Lutrate Depot
• Your condition may get worse at first during the first weeks of the treatment, but should improve with continued treatment. Such signs and symptoms include: temporary increase of testosterone (a male hormone), hot flushes, bone pain, nervous system disorders (including depression) or urinary obstruction.
• If you feel you have experienced an allergic reaction (shortness of breath, asthma, rhinitis, swelling of the face, urticaria, skin eruption), stop using this medicine and inform your doctor.
• Tell your doctor if you might be at risk or if you have any of the following as you may need more frequent check ups:
• you suffer from any unexplained bruising or bleeding or if you feel generally unwell. Although rare, these could be symptoms of changes in the number of red or white cells
• you have metabolic disease
• you have heart problems, or a pounding heart beat
• you have diabetes
• Your doctor should be aware of any previous personal clinical history of pituitary adenoma (non-cancerous tumor of pituitary). Cases of pituitary apoplexy (partial tissue loss of pituitary gland) have been described after initial administration of this type of drug to patients with pituitary adenoma. Pituitary apoplexy may be manifested by sudden headache, meningismus, visual disturbances or altered vision, even blindness, and occasionally, decrease in the level of consciousness.
• Your doctor should be aware if you suffer from a bleeding disorder, thrombocytopenia or if you are on treatment with anticoagulants. Your liver function may need to be monitored as changes to the liver and jaundice (yellow eyes and skin) have been reported with leuprorelin treatment.
• A fractured spine, paralysis, low blood pressure and high blood pressure have been reported with leuprorelin treatment.
• There have been reports of depression in patients taking Lutrate Depot which may be severe. If you are taking Lutrate Depot and develop depressed mood, inform your doctor.
• A decreased bone density (brittleness or thinning of the bones) has been reported with leuproreline. Your doctor may consider adding an antiandrogen to the treatment with Lutrate Depot. Your doctor will be on the alert for inflamed veins (thrombophlebitis) and other signs of clotting disorders and oedema (swelling of hands, feet or ankles). There is an increased risk of these occurring in the case that an antiandrogen treatment is added to Lutrate Depot.
• Tell your doctor, if you feel pressure on the spinal cord and/or experience urinary disorders and/or haematuria (blood in the urine). In this case your doctor will be take if necessary, additional precautions to avoid neurological complications (e.g. tingling in hands and feet, paralysis) or obstruction of the urethra (the tube that connects the bladder to the outside of the body). You will be closely supervised during the first weeks of treatment.
• Patients may experience metabolic changes (e.g. glucose intolerance or worsening of existing diabetes), weight changes and cardiovascular disorders.
• Patients with metabolic or cardiovascular disease and especially patients with history of congestive heart failure (condition in which the heart can no longer pump enough blood to the rest of the body) should be monitored during treatment with leuprorelin.
• You will need some blood tests during treatment, to check that Lutrate Depot is being efficacious.
• You may experience a loss of interest in sexual intercourse, hot flushes and occasionally there may be a reduction in size and function of the testes.
• You may become fertile again when Lutrate Depot treatment is stopped.
• Lutrate Depot may interfere with certain laboratory tests so make sure your doctor knows you are using Lutrate Depot
• Convulsions can occur in predisposed patients (those with a history of seizures, epilepsy, cerebrovascular disorders, anomalies or central nervous system tumors), in patients receiving drugs that can cause seizures and, to a lesser extent, in other patients who do not have these characteristics.
• Please tell your doctor if you have any of the following: Any heart or blood vessel conditions, including heart rhythm problems (arrhythmia), or are being treated with medicines for these conditions. The risk of heart rhythm problems may be increased when using Lutrate Depot.
Other medicines and Lutrate Depot
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. It may still be all right for you to be given Lutrate Depot and your doctor will be able to decide what is suitable for you.
Lutrate Depot might interfere with some medicines used to treat heart rhythm problems (e.g. quinidine, procainamide, amiodarone and sotalol) or might increase the risk of heart rhythm problems when used with some other drugs(e.g. methadone (used for pain relief and part of drug addiction detoxification), moxifloxacin (an antibiotic), antipsychotics used for serious mental illnesses).
Pregnancy and breast-feeding
Lutrate Depot is not indicated for use in women.
This medicine is contraindicated during pregnancy. Spontaneous abortions may occur if this medicine is administered during pregnancy.
Driving and using machines
No specific studies on the effects of Lutrate Depot on the ability to drive and use machines have been performed.
Disturbance of vision and dizziness can occur during treatment. If affected you should not drive or operate machinery.
Lutrate Depot contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
Dose
Lutrate Depot must be given under the supervision of a doctor or a qualified health practitioner.
Adults including the elderly:
The recommended dose of Lutrate Depot is an injection once every three months. The powder is made up into a suspension and given as a single injection intramuscularly (into a muscle) once every three months The injection site should be varied at regular intervals.
Lutrate Depot must be administered via the intramuscular route only. Do not administer by another route.
Use in children: Lutrate Depot is not indicated for use in children.
The strength of your treatment is decided by your doctor.
If you use more Lutrate Depot than you should
This is unlikely as your doctor or nurse will know the correct dosage. However, if you suspect you have received more than you should, let your doctor know about it immediately so appropriate measures can be taken.
If you forget a dose of Lutrate Depot
It is important not to miss a dose of Lutrate Depot. As soon as you realise you have missed an injection contact your doctor who will be able to give you your next injection.
If you stop using Lutrate Depot
Since the medical treatment involves administration of Lutrate Depot for a long period, when the treatment is interrupted you may experience a worsening of the symptoms related to the disease. Therefore you must not interrupt the treatment prematurely without your doctor’s permission.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them. Tell your doctor straight away if you get any sudden wheeziness, difficulty in breathing, swelling of the eyelids, face or lips, rash or itching (especially affecting your whole body).
The following side effects have been reported:
Very common (These may affect more than 1 in 10 people):
Hot flushes and injection site reactions.
Common (These may affect up to 1 in 10 people):
Cold sweats, hyperhidrosis (increased sweating), pruritus (itching), fatigue, insomnia (not sleeping), decreased sex drive, spinning sensation (dizziness), flushing, feeling sick (nausea), diarrhoea, , decreased appetite, erectile dysfunction, asthenia (lack or loss of strength), bone pain, pain in the joints and injection site reactions such as pain, induration, erythema (redness of the skin). Urinary tract pain, urine flow decreased, frequent need to urinate, mood changes and depression in long term use of leuprorelin, changes in liver enzymes and, blood triglyceride increased (high levels of blood lipids), blood suggar increased.
Uncommon (These may affect up to 1 in 100 people):
High cholesterol, sleep disorders, feeling jittery, taste disturbance, formication (alteration in the skin sensation), headache, lethargy (sleepiness), vision blurred, pleurisy, ringing in the ears (tinnitus), tummy pain upper, constipation, papule, rash, pruritus generalised (itching), night sweats, back pain, muscle aching, neck pain, nipple pain, pelvic pain, testicular atrophy, testicular disorder, feeling hot, mood changes depression in short term use of leuprorelin. Changes in blood values and changes in ECG (QT prolongation). And injection site reactions such as: urticaria, warmth and hemorrhage.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card Scheme website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
Your doctor or pharmacist will know how to store Lutrate Depot.
Keep this medicine out of sight and reach of children.
Do not store above 25o C. Do not freeze.
Do not use this medicine after the expiry date which is stated on the box, vial and pre-filled syringe after "EXP". The syringe has the same expiry date to that of the vial. The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Lutrate Depot contains
The active substance is leuprorelin acetate. Each vial contains 22.5 mg of leuprorelin acetate.
The concentration of the reconstituted product is 11.25 mg/ml.
The other ingredients are: Polysorbate 80, Mannitol (E-421), Carmellose sodium (E-466), Triethyl citrate and Poly(lactic acid) (PLA).
The solvent contains (pre-filled syringe): mannitol, water for injection, sodium hydroxide (for pH adjustment) and hydrochloric acid (for pH adjustment).
What Lutrate Depot looks like and contents of the pack
Each pack contains a vial with 22.5 mg of leuprorelin acetate, one prefilled syringe with 2 ml of solvent, one adaptor system and one sterile 20 gauge needle.
Marketing Authorisation Holder and Manufacturer
GP-PHARM, S.A.
Pol ind Els Vinyets -els Fogars. Sector 2 Carretera comarcal 244, km22 08777 Sant Quint! de Mediona.
Spain
This medicinal product is authorised in the Member States of the EEA under the following names:
Spain: Lutrate Depot 22.5 mg polvo y disolvente para suspension de liberacion prolongada inyectable
Germany: Lutrate Depot 22.5 mg pulver und losungsmittel zur Herstellung einer Depot-I njektionssuspension
Portugal: Lutrate Depot 22.5 mg / 2 ml po e velculo para suspensao injectavel de libertagao prolongada
Greece: Lutrate Depot 22.5 mg Kovig Kai 5iaAuTng Yia napaoKEup evsaipou £vaiwpppaTog napaT£Tap£vpg ano5£ap£uang
Italy: Politrate 22.5 mg Polvere e solvente per sospensione iniettabile a rilascio prolungato.
Sweden: Politrate 22.5 mg pulver och vatska till injektionvaska, suspension
Hungary: Politrate Depot 22.5 mg
Denmark: Lutrate Depot
Finland: Lutrate 22.5 mg
Ireland: Lutrate Depot 22.5 mg powder and solvent for prolonged-release suspension for injection
United Kingdom: Lutrate Depot 22.5 mg powder and solvent for prolonged-release suspension for injection
Belgium: Lutrate Depot 22.5 mg poeder en oplosmiddel voor suspensie voor injectie met verlengde afgifte
The Netherlands: Leuproreline Lutrate Depot 22,5 mg poeder en oplosmiddel voor suspensie voor injectie met verlengde afgifte Norway: Lutrate Depot
Austria: Lutrate Depot 22.5 mg pulver und losungsmittel zur herstellung einer Depot-
injektionssuspension
Estonia: Lutrate Depot 22.5 mg
Lithuania: Lutrate Depot 22.5 mg milteliai ir tirpiklis pailginto atpalaidavimo injekcinei
Latvia: Lutrate Depot 22.5 mg pulveris un skldinatajs ilgstosas darblbas injekciju suspensijas
pagatavosanai
Czech Republic: Lutrate Depot 22.5 mg
Poland: Lutrate Depot
Slovak Republic: Lutrate Depot 22.5 mg
Romania: Lutrate Depot 22.5 mg pulbere §i solvent pentru suspensie injectabila cu eliberare prelungita
Bulgaria: Lutrate Depot
This leaflet was last revised in 05/2015.
The following information is intended for healthcare professionals only:
How to prepare the injection?
Follow these instructions carefully.
An aseptic technique should be observed during the reconstitution procedure.
Important:
Once mixed, the product must be administered immediately.
This product is for single use only.
Verify the contents of the kit and make sure it includes everything that’s mentioned in the leaflet.
The pack contains:
1 (one) vial of Lutrate Depot 22.5 mg (leuprorelin acetate) powder for suspension for injection 1 (one) pre-filled syringe containing the suspension solvent (mannitol 0.8% solution for injection);
1 (one) device for reconstitution containing 1 (one) single-use sterile needle.
1

8
Remove cap from the vial
4
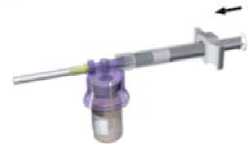
While keeping the syringe and vial securely coupled in an upright position, slowly push the plunger in order to transfer all the diluent into the vial
2

Attach the adaptor system (in purple) to vial until a ‘clicking’ sound is heard
5
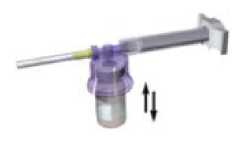
With the syringe still coupled to the vial, shake the vial gently for approximately one minute until a uniform milky-white suspension is obtained
8
3

Affix the white finger-grip to the diluents-containing syringe.
Remove the rubber cap from the syringe and attach it to the adaptor system
6

Turn the system upside down, and carefully pull out the plunger to draw-up the resuspended drug from the vial into the syringe

Detach the syringe and needle from the adaptor system by twisting the upper piece of the adaptor counter-clockwise. The drug is ready to be used.
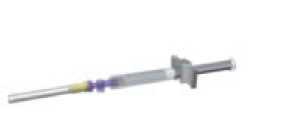
Clean the injection area with an alcohol swab and let the skin dry.
Inject the suspension intramuscularly into the upper outer quadrant of the gluteus
Some product may cake or clump at the vial wall. This is considered normal. During the manufacture of the product the vial is filled with excess product in order to make sure that a final dose of 22.5 mg of leuprorelin acetate is administered.