Magnevist 2 Mmol/L Solution For Injection
#

Package Leaflet: Information for the user
Magnevist® 2 mmol/l solution for injection
dimeglumine gadopentetate

dimeglumine gadopentetate
Summary of Product Characteristics
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
► Keep this leaflet. You may need to read it again.
► If you have any further questions, please ask the doctor giving you Magnevist 2 mmol/l (the radiologist) or the hospital/MRI-centre staff.
► If you get any side effects, talk to your doctor, radiologist or the hospital/MRI-centre staff.This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Magnevist 2 mmol/l is and what it is used for
2. What you need to know before you are given Magnevist 2 mmol/l
3. How you will be given Magnevist 2 mmol/l
4. Possible side effects
5. How to store Magnevist 2 mmol/l
6. Contents of the pack and other information
1. What Magnevist 2 mmol/l is and what it is used for
Magnevist 2 mmol/l is used together with a technique called Magnetic Resonance Imaging (MRI) to create artificial contrast or enhancement to help make an MRI scan of your joints clearer.
MRI is a modern scanning technique which produces very high quality pictures ofvarious parts of your body without using X-rays. The use of MRI can provide a quick, early and accurate diagnosis.
The scanner uses a strong magnetic field and radiowaves to measure the magnetic properties of body tissues. Using a computer, this information is converted into a black and white picture which can help your doctor see and investigate the differences between normal and abnormal tissue.
Sometimes MRI is used in areas where it cannot produce a clear black and white picture. This is when Magnevist 2 mmol/l is used. Magnevist 2 mmol/l produces a clearer image and allows the doctor to see the area of interest better. Sometimes several scans will be taken before Magnevist 2 mmol/l is injected and then further scans taken after the injection.
This medicine is for diagnostic use only.
2. What you need to know before you are given Magnevist 2 mmol/l
Do not use Magnevist 2 mmol/l:
► if you are allergic to dimeglumine gadopentetate or any of the other ingredients of this medicine (listed in section 6)
► if you have a heart pacemaker or if there are any implants or clips containing iron inside your body. (This is not because of an interaction with Magnevist 2 mmol/l but because if you have any of these, you should not be placed in a strong magnetic field).
Warnings and precautions
Talk to your radiologist or MRI-centre staff before receiving
Magnevist 2 mmol/l
Your doctor will need to take special care when giving you
Magnevist 2 mmol/l if:
► you have a history of allergy (e.g. hay fever, hives), asthma, or have had a reaction to another type of contrast media.
This is because you may be more likely to have an allergic reaction. If you have had any of these in the past, tell the radiologist or MRI-centre staff.
► you have an infected joint.
Before you receive Magnevist 2 mmol/l, you must tell the
radiographer or MRI-centre staff if any of these apply to you.
Other medicines and Magnevist 2 mmol/l
Tell the radiologist or MRI-centre staff ifyou are taking, have
recently taken or might take any other medicines.
Pregnancy and breast-feeding
Ifyou are pregnant or breast-feeding, thinkyou may be pregnant or are planning to have a baby, ask the MRI-centre staff for advice before receiving this medicine. A very small amount of Magnevist enters the breast milk, but this isn’t likely to cause any harm.
Driving and using machines
The volume of Magnevist 2 mmol/l injected into your joint may limit your movement. Do not drive if your movement is affected.
Magnevist 2 mmol/l contains sodium
This medicinal product contains 3.4 mg sodium per ml. To be taken into consideration by patients on a controlled sodium diet.
B. How you will be given Magnevist 2 mmol/l
You will be asked to sit or lie down and Magnevist 2 mmol/l will be injected into the joint which is being investigated. Scanning may start immediately after the Magnevist 2 mmol/l injection. The MRI staff will observe you for at least 30 minutes after the injection just in case you have any side effects.
Adults:
The dose of Magnevist 2 mmol/l varies depending on which joint is investigated. For most joints, 20 ml is used, but up to 50 ml may be used for the knee joint. The doctor will decide how much Magnevist 2 mmol/l is needed for your investigation. Children:
Magnevist 2 mmol/l is not recommended for use in children. Ifyou receive more Magnevist 2 mmol/l than you should
Overdosing is unlikely. If this does occur no serious ill-effects are expected.
Ifyou have any further questions on the use of this medicine, ask the MRI-centre staff.
Please turn over
1. NAME OF THE MEDICINAL PRODUCT
Magnevist 2 mmol/l solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
1 ml aqueous solution contains 1.876 mg gadopentetic acid, dimeglumine salt as active ingredient (equivalent to 0.002 mmol gadopentetic acid, dimeglumine, containing 0.32 mg gadolinium).
Excipient with known effect: sodium chloride Magnevist 2 mmol/contains 3.4 mg sodium per ml.
For the full list of excipients, see section 6.1.
B. PHARMACEUTICAL FORM
Solution for injection.
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
For contrast enhancement in direct magnetic resonance arthrography.
This medicinal product is for diagnostic use by intraarticular administration only.
4.2 Posology and method of administration
The usual precautions for MRI (e.g. exclusion of cardiac pacemakers and other ferro-magnetic objects including vascular clips etc) must be observed.
Posology
The recommendations for the use of Magnevist 2 mmol/l apply to a field strength between 0.2 Tesla and 1.5 Tesla. Intraarticular administrations of contrast agents are to be given with the patient lying or sitting. After the end of the injection, the patient should be kept under supevision for at least half an hour.
Adults:
In general, for all joints the administration of up to 20 ml (knee joint up to 50 ml) Magnevist 2 mmol/l is sufficient for good opacification and to answer all the relevant clinical questions.
A volume leading to a slight distension of the joint capsule should be injected. Only so much contrast medium should be injected until discrete resistance is felt and/or the patient experiences a mild feeling of pressure.
Guidelines on volumes to be administered:
|
Joint |
Volume required |
|
Shoulder |
15-20 ml |
|
Elbow |
~ 10 ml |
|
Wrist |
4 ml |
|
Finger joint |
1-2 ml |
|
Hip |
10-20 ml |
|
Knee |
25-50 ml |
|
Ankle |
12-20 ml |
Paediatric population:
The safety and efficacy of Magnevist 2 mmol/l in children aged up to 18 years has not yet been established. No data are available. Magnevist 2 mmol/l is not recommended in the paediatric age group until further data become available. Method of administration
The dose required is administered via intraarticular injection under strict aseptic technique and according to the instructions provided in section 6.6.
Contrast-enhanced MRI can be commenced immediately afterwards.
4.4 Special warnings and precautions for use
Strict aseptic technique is required to prevent infection. Fluoroscopic control should be used to ensure proper needle placement and prevent extracapsular injection. Undue pressure should not be exerted during injection.
Intraarticular injections of Magnevist 2 mmol/l should be avoided in infected joints.
► Flypersensitivity
Severe systemic hypersensitivity reactions cannot be totally excluded (see section 4.8).
Mild angioedema, conjunctivitis, coughing, pruritus, rhinitis, sneezing and urticaria, which can occur irrespective of the amount administered and the mode of administration, may be the first signs of incipient state of shock.
As with other contrast agents, delayed reactions may occur (hours later or up to several days).
As with other contrast enhanced diagnostic procedures, postprocedure obsevation of the patient is recommended.
Medication for the treatment of hypersensitivity reactions as well as readiness for institution of emergency measures are necessary. Appropriate drugs and instruments (e.g. endotracheal tube and ventilator) must be readily available.
The risk of hypersensitivity reactions is higher in case of:
► previous reaction to contrast media,
► history of bronchial asthma,
► history of allergic disorders
The decision to use Magnevist 2 mmol/l must be made after particularly careful evaluation of the risk-benefit-ratio in patients with an allergic disposition.
► Magnevist 2 mmol/l contains sodium
This medicinal product contains 3.4 mg sodium per ml. To be taken into consideration by patients on a controlled sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction
As for all other gadolinium containing contrast media, no interactions with other medicaments have been observed.
Formal drug interaction studies have not been carried out. See also section 6.2.
Magnevist should be administered without the addition of iodinated contrast media as iodinated contrast media reduce the level of contrast achievable with Magnevist (see section 6.6).
4.6 Fertility, pregnancy and lactation
► Pregnancy
For gadopentetic acid, dimeglumine no clinical study data on exposed pregnancies are available. Animal studies do not indicate direct or indirect harmful effects with respect to embryonal / foetal development (see section 5.3).
Caution should be exercised using Magnevist 2 mmol/l in pregnant women.
► Breast-feeding
No data exist concerning intra-articular administration in lactating women. After intravascular use minimal amounts of gadopentetic acid, dimeglumine salt (a maximum of 0.04%) of the intravenously administered dose enters the breast milk.
From experience gained so far, harm to the breast-fed infant is considered unlikely.
4.7 Effects on ability to drive and use machines
No effects of Magnevist 2 mmol/l on driving ability and use of machinery can be expected. However, joint effusion may affect the ability to drive due to a limited joint mobility.
4.8 Undesirable effects
Frequency of adverse reactions from clinical trial data
Based on experience in more than 4,900 patients, the undesirable effects listed below have been observed and classified by investigators as drug-related.
Adverse reactions with the use of Magnevist 2 mmol/l are usually of mild to moderate intensity.
The most frequently reported reactions were local injection site reactions, i.e. injection site pain and joint pressure sensations which are mainly related to the procedure itself.
The table below reports adverse reactions by MedDRA system organ classes (MedDRASOCs).
|
System Organ Class |
Common (>1/100 to <1/10) |
Uncommon (>1/1,000 to <1/100) |
Rare (>1/10,000 to <1/1,000) |
|
Nervous system disorders |
Headache Dizziness | ||
|
Vascular disorders |
Vasovagal reaction | ||
|
Gastrointestinal disorders |
Nausea |
Vomiting | |
|
General disorders and administration site conditions |
Injection site pain/ Injection site (joint) pressure sensation |
The most appropriate MedDRA term is used to describe a reaction and its synonyms and related conditions.
► Immune system disorders/Hypersensitivity/Allergic reaction Systemic hypersensitivity may occur rarely in the form of skin reactions. The possibility of a severe hypersensitivity reaction cannot be totally excluded (see section 4.4).
► General disorders and administration site conditions Injection of Magnevist 2 mmol/l into the joint is commonly associated with transient discomfort, e.g. pressure and pain due to the injected volume. Severe pain may often result from undue use of pressure or the injection of large volumes.
Other adverse reactions commonly known from intravenous injection of gadolinium chelates were so far not observed with Magnevist 2 mmol/l, due to the low dose and the topical administration.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important, it allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No signs of intoxication secondary to an overdose have so far been obseved or reported on clinical use.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: paramagnetic contrast media,
ATC code: V08CA01
Magnevist 2 mmol/l is a paramagnetic contrast agent for magnetic resonance imaging. The contrast-enhancing effect is mediated by the di-N-methylglucamine salt of gadopentetic acid, dimeglumine - the gadolinium complex of pentetic acid (diethylene triamine pentaacetic acid = DTPA). When a suitable scanning sequence (e.g. Tj-weighted spin-echo technique) is used in proton magnetic resonance imaging, the gadolinium ion-induced shortening of the spin-lattice relaxation time of excited atomic nuclei leads to an increase of the signal intensity and, hence, to an increase of the image contrast of certain tissues.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Please turn over
Gadopentetic add, dimeglumine is a highly paramagnetic compound which leads to distinct shortening of relaxation times, even in low concentrations. The paramagnetic efficacy, the relaxivity (determined from the influence on the spin-lattice relaxation time of protons) is 3.67 in water and about 4.95 l/mmol/sec in plasma, and displays only slight dependency on the strength of the magnetic field.
The concentration of Magnevist 2 mmol/l corresponds to 1/250 of the concentration used for i.v. administration. This concentration is sufficient to allow adeguate imaging efficacy even after further dilution with joint effusion. If the joint cavity is filled with gadolinium-containing fluid, the signal in the cavity increases on use of Tj-weighted seguences, i.e. it becomes bright and contrasts clearly with all structures with a weak or intermediate signal (i.e. all intraarticular structures: hyaline and fibrous cartilage, all ligaments, tendons and the joint capsule). While normal, or even increased, joint fluid does not differ in its signal behaviour in Tj-weighted images from all the other anatomical structures apart from fibrocartilage, the intraarticular administration of Magnevist 2 mmol/l leads to distinctly improved contrast situations.
DTPA forms a firm complex with the paramagnetic gadolinium ion with extremely high in vivo and in vitro stability (log K = 22 - 23). The dimeglumine salt of gadopentetic acid, dimeglumine is a highly water-soluble, extremely hydrophilic compound with a distribution coefficient between n-butanol and buffer at pH 7.6 of about 0.0001. The substance does not display any particular protein binding or inhibitory interaction with enzymes (e.g. myocardial Na+and K+ATPase). Magnevist 2 mmol/l does not activate the complement system and, therefore, probably has a very low potential for inducing anaphylactoid reactions.
Based on clinical experience, impairment of hepatic, renal or cardiovascular function is not expected.
The physico-chemical properties of Magnevist 2 mmol/l listed below are:
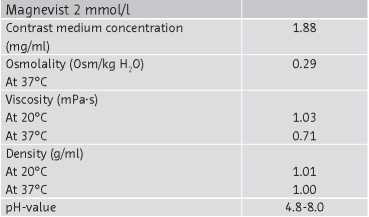
5.2 Pharmacokinetic properties
The pharmacokinetic properties of gadopentetic acid, dimeglumine have been extensively studied after intravenous and oral administration in doses exceeding the amount injected intraarticularly.
After intraarticular injection the compound distributes in the synovial fluid and diffuses into the interstitial space. Marginal uptake into the cartilage is completely reversible.
After distribution in the extracellular space primarily through diffusion controlled processes, the gadopentetic acid, dimeglumine is eliminated unmetabolised via the kidneys by glomerular filtration.
5.3 Prectinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of systemic toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and contact sensitising potential.
► Local tolerance
Experimental local tolerance studies with gadopentetic acid, dimeglumine (at a concentration of 500 mmol/l) following single subcutaneous and intramuscular administration in animals indicated that slight local intolerance reactions could occur at the injection site after inadvertent administration.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
pentetic acid meglumine sodium chloride water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life 3 years
6.4 Special precautions for storage
None
6.5 Nature and contents of container
Colourless Type I, glass pre-filled syringe with chlorinated butyl rubber stopper and combined luer lock adapter, tip cap (chlorobutyl rubber), safety cap.
Pack size: syringe containing 20 ml of Magnevist solution; individual syringe is blister packaged, with one syringe per carton.
6.6 Special precautions for disposal and other handling
The prefilled syringe must be taken from the pack and prepared for the injection immediately before the examination and injected under sterile conditions.
The tip cap should be removed from the prefilled syringe immediately before use.
Any contrast medium solution not used in one examination must be discarded.
Mixture of Magnevist 2 mmol/l with X-ray contrast media before injection is not recommended as it may reduce efficacy. The minimal amount of X-ray contrast medium reguired for control of the needle position in the joint may be separately injected prior to the administration of Magnevist 2 mmol/l (0.5 ml to a maximum of 1.0 ml).
7. MARKETING AUTHORISATION HOLDER
Bayer pic Bayer House Strawberry Hill Newbury Berkshire RG14 1JA
8. MARKETING AUTHORISATION NUMBER
PL 00010/0544
9. DATE OF FIRST AUTHORISATION / RENEWAL OF THE AUTHORISATION
01 May 2008/23 February 2009
10. DATE OF REVISION OF THE TEXT
to be updated once the variation is approved Legal category: POM
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects you may get after being given a contrast medium like Magnevist 2 mmol/l are usually mild to moderate.
Ifyou notice:
► itching of the skin, rash, wheals on the skin (urticaria)
► difficulty breathing, gagging, feeling of suffocation
► swelling of the face, neck or body
► itchy or watery eyes, tickling in the throat or nose, hoarseness, coughing or sneezing
► headache, dizziness, feeling faint
► feeling particularly hot or cold, sweating
► paleness or reddening of the skin
► chest pain, cramp, tremor
► feeling sick
Tell the radiologist or MRI staff immediately as these may be the first signs of allergic reaction or shock. Your investigation will need to be stopped and you may need further treatment. Apart from the symptoms listed above, these are the possible side effects of Magnevist 2 mmol/l, starting with the more common ones:
Common
may affect up to 1 in 10 people
► sensations or reactions at the injection site (such as pain, joint pressure and temporary discomfort)
Uncommon
may affect up to 1 in 100 people
► feeling sick
► dizziness
► headache Rare
may affect up to 1 in 1,000 people
► being sick
► slowed heartbeat; lowered blood pressure; fainting
► allergic-type skin reactions including itching, redness, wheals on the skin
Delayed reactions can occur (after hours or days), ifyou are concerned you should contact your doctor.
Reporting of side effects
Ifyou get any side effects, talk to your radiologist or MRI-centre staff. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Magnevist 2 mmol/l
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after EXP. The expiry date refers to the last day of that month.
6. Contents of the pack and other information
What Magnevist 2 mmol/l contains
► The active substance is dimeglumine gadopentetate.
1 ml Magnevist 2 mmol/l contains 1.876 mg of the dimeglumine salt of gadopentetic acid.
► The other ingredients are meglumine, pentetic acid, sodium chloride and water for injections.
What Magnevist 2 mmol/l looks like and contents of the pack
Magnevist 2 mmol/l is available in 20 ml pre-filled syringes.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Bayer pic Bayer House Strawberry Hill Newbury Berkshire RG14 1JA Manufacturer:
Bayer Pharma AG Berlin
This leaflet was last revised in April 2015,
To listen to or reguest a copy of this leaflet in Braille, large print or audio please call, free of charge:
0800 198 5000 (UK only)
Please be ready to give the following information:
|
Product name |
Reference number |
|
MAGNEVIST® 2 mmol/l solution for injection |
00010/0544 |
This is a service provided by the Royal National Institute of the Blind.
Magnevist® 2 mmol/l solution for injection


Bayer
84618314