Medical Carbon Dioxide
PRODUCT SUMMARY
1. NAME OF THE MEDICINAL PRODUCT Medical Carbon Dioxide
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Medical Carbon Dioxide specification is:
Carbon Dioxide Purity 99.5% v/v min
The Medical Carbon Dioxide cylinder specification complies with the current European Pharmacopeia monograph (0375)
3 PHARMACEUTICAL FORM
Medicinal gas, liquefied 4. CLINICAL PARTICULARS
4.1. Therapeutic indications
Carbon dioxide is used;
• to increase depth of anaesthesia rapidly when volatile agents are being administered. It increases depth of respiration and helps to overcome breathholding and bronchial spasm
• to facilitate blind intubation in anaesthetic practice
• to facilitate vasodilation and thus lessen the degree of metabolic acidosis during the induction of hypothermia
• to increase cerebral blood flow in arteriosclerotic patients undergoing surgery
• to stimulate respiration after a period of apnoea
• in chronic respiratory obstruction after it has been relieved
• to prevent hypocapnia during hyperventilation
• for clinical and physiological investigations
• in gynaecological investigation for insufflation into fallopian tubes and abdominal cavities as solid Carbon Dioxide (dry ice) in tissue freezing techniques and for the destruction of warts by freezing
4.2. Posology and method of administration
Carbon Dioxide is usually administered through the lungs by inhalation. The major exceptions are when a metered supply is fed into the oxygenator of an extracorporeal circulation of a cardio-pulmonary by-pass system, and when the gas is used for laparoscopic surgery.
There are no distinctions between the use of Carbon Dioxide in any age group.
Carbon Dioxide should only be given under the direct supervision of a clinician. Except under special circumstances (e.g. physiological investigations), the inspired concentration should not exceed 5%. However, 100% Carbon Dioxide may be insufflated into the abdominal cavity to distend it to allow the investigation and treatment of intra-abdominal disease, particularly of a gynaecological nature.
4.3. Contraindications
Carbon Dioxide is contra-indicated:
• in acidosis
• in respiratory obstruction, the administration of Carbon Dioxide may be dangerous since any further increase in respiratory effort increases negative intra-thoracic pressure
• during resuscitation, where it can be dangerous and should be avoided
4.4. Special warnings and precautions for use
Carbon Dioxide is stored in high pressure gas cylinders as a liquid under pressure. Rapid opening of the valve can cause the discharged gas to re--liquefy. This liquid can cause cold burns if in contact with the skin. Cylinders should only be used in the vertical position with the valve uppermost.
Care is needed in the handling and use of Carbon Dioxide gas cylinders.
4.5.
Interaction with other medicinal products and other forms of interaction
Carbon Dioxide interacts with anaesthetic agents when the concentration is raised and gives rise to cardiac dysrhythmias. The threshold for dysrhythmias varies with different anaesthetic drugs.
Carbon Dioxide, by altering pH, influences uptake distribution and action of many drugs including neuromuscular blocking agents, and hypotensive agents.
Carbon Dioxide interacts with adrenergic substances such as adrenaline. They should not be used together.
4.6. Pregnancy and lactation
The use of Carbon Dioxide is not recommended in pregnancy but is unlikely to influence lactation.
4.7. Effects on ability to drive and use machines
Inhalation of Carbon Dioxide is not compatible with driving or use of machinery.
4.8 Undesirable effects
Carbon Dioxide may produce unconsciousness in concentrations over 10%. Cardiac dysrhythmias have been reported in patients undergoing laparoscopy as a result of high blood Carbon Dioxide levels. Cardiac arrest due to gas embolism has been reported.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via The Yellow Card System www.mhra.gov.uk/yellowcard
4.9. Overdose
Moderate overdose of Carbon Dioxide less than 5% stimulates breathing. If excessive this may cause extreme respiratory difficulty, raise the blood pressure and lead to nausea and vomiting and occasionally unconsciousness. In concentrations above 10%, Carbon Dioxide possesses anaesthetic properties.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group: Medical Gases ATC Code: V03AN02
The characteristics of Carbon Dioxide are:
• odourless, colourless gas
molecular weight sublimation point density
44.00
-78.5oC(at 1 bar) .
1.872 kg/m3 (at 15°C)
Carbon Dioxide occurs at approximately 350 vpm in the atmosphere.
The effect of inhaling Carbon Dioxide, or of its accumulation in the body through breathing defects, varies with the tension achieved in the blood, the duration and condition of the exposure and the susceptibility of the individual concerned.
If a normal, conscious individual inhales 5% Carbon Dioxide, the rate and depth of breathing rise and the minute volume increases 2 to 5 fold. The skin becomes pink and warm and there may be sweating and a sense of discomfort. There is no effect on consciousness or mental function, even with long exposures. After a prolonged exposure when the return to breathing air takes place, an "off effect" may develop with malaise, pallor, headache and occasional nausea and vomiting, probably due to the metabolic disturbances as a result of breathing a volatile acid.
As the inspired concentration rises, these effects become exaggerated in proportion to the concentration. At around 8-9% dizziness may develop, and at 10% some subjects become unconscious. Most people will become unconscious at 12.5% and all subjects lose consciousness within 1-2 minutes at 20%. When the concentration is raised to 30% consciousness is lost rapidly, the blood pressure may rise to 27 kpa (200 mm Hg) or higher and there is intense vasoconstriction, a reduction in heart rate to 40-50 heats per minute and ECG changes. All anaesthetic agents reduce these responses to Carbon Dioxide.
5.2. Pharmacokinetic properties
When inhaled, Carbon Dioxide is rapidly distributed throughout the body. Physiologically, it regulates the rate and depth of breathing and normally there is a constant tension of 5 kpa (40 mm Hg) in arterial blood. The concentration of Carbon Dioxide in the plasma is three times greater than that in red blood cells. The gas is carried partly in solution (2.4 - 2.7 vol %), but mostly either as bicarbonate (42.9 - 46.7 vol %), or as carbamino compound (3.0 - 3.7 vol %). The relative quantities in solution and as bicarbonate regulate the reaction of the blood and buffer changes in pH produced by stronger organic acids.
Carbon Dioxide produced by metabolism plays an integral part in the supply of oxygen to the tissues, since the amount releases by haemoglobin at any given oxygen tension is directly related to the Carbon Dioxide tension in the blood. This in turn is governed by tissue activity in the concentration inhaled.
Thus the rate at which oxygen is given up to the tissues is increased when the Carbon Dioxide tension is raised.
When a patient becomes apnoeic, Carbon Dioxide produced in the tissues, accumulates in blood at a rate of about 0.7 kpa (5 mm Hg) per minute.
5.3. Preclinical safety data
None stated
6. PHARMACEUTICAL PARTICULARS 6.1. List of excipients
Inert gases
6.2. Incompatibilities
Carbon dioxide should not be given when adrenaline is used.
6.3. Shelf life
36 months
6.4 Special precautions for storage
Carbon Dioxide cylinders should be:
• stored under cover, preferably inside, kept dry and clean, and not subjected to extremes of heat or cold and away from stocks of combustible material.
• stored separately from industrial and other non-medical cylinders.
• stored to maintain separation between full and empty cylinders.
• used in strict rotation so that cylinders with the earliest filling date are used first.
• stored separately from other medical cylinders within the store
Warning notices prohibiting smoking and naked lights must be posted clearly in the cylinder storage area and the Emergency Services should be advised of the location of the cylinder store.
Care is needed when handling and using Medical Carbon Dioxide cylinders.
6.5 Nature and contents of container
A summary of Medical Carbon Dioxide cylinders, their size and construction, type of valve fitted is detailed below:
|
Cylinder Size |
Gas Content (litres) |
Cylinder Construction |
Valve Outlet |
Valve Construction |
|
C |
450 |
Steel |
Pin Index |
Brass |
|
E |
1800 |
Steel |
Pin Index |
Brass |
|
VF |
3600 |
Steel |
0.860” X 14 TPI (M) |
Brass |
|
LF |
3600 |
Steel |
0.860” X 14 TPI (M) |
Brass |
Cylinders
All cylinders used for the storage of Carbon Dioxide are manufactured from high tensile steel with a designed working pressure of at least 137 bar g. All cylinders require an appropriate regulator to be fitted prior to use.
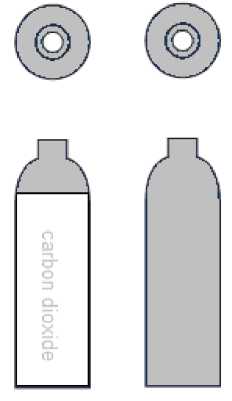
The colour coding of the shoulders of Medical Carbon Dioxide is grey (RAL 7037).
The colour coding of the cylinder body is white (RAL 9010). Cylinders also carry the carbon dioxide name on the body of the cylinder.
For a limited period, cylinders may have grey bodies. These cylinders do not have the name carbon dioxide on the body of the cylinder.
The programme to convert all Medical Carbon dioxide cylinders to white bodies will be completed by 2025.
Cylinder Valves
Medical Carbon Dioxide cylinders are supplied with two main types of cylinder valves.
Medical Carbon Dioxide C and E cylinders are fitted with valves with outlet connections that conform to ISO 407 (pin index).
LF and VF size cylinders are fitted with outlet connections that conform to BS 341(Type 8) (11/16" x 20 TPI (M)) and are filled with liquid to a specified weight. The pressure in the cylinder is dependant on the vapour pressure at the cylinder temperature.
The cylinder valves are constructed from high tensile brass with a steel spindle fitted with a Nylon 6.6 insert.
6.6 Special precautions for disposal (and other handling)
All personnel handling Carbon Dioxide cylinders should have adequate knowledge of:
• properties of the gas
• correct operating procedures for the cylinder
• precautions and actions to be taken in the event of an emergency.
Preparation for Use
To prepare the cylinder for use:
• remove the tamper evident seal and the valve outlet protection cap. Ensure cap where fitted, is retained so that it can be refitted after use.
• Do not remove and discard any batch labels fitted to the cylinder.
• ensure that an appropriate regulator is selected for connection to the cylinder.
• ensure the connecting face on the regulator is clean and the sealing washer fitted is in good condition.
• connect the regulator, using moderate force only and connect the tubing to the regulator / flowmeter outlet. Only the appropriate regulator should be used for the particular gas concerned.
• Ensure that the cylinder valves and any associated equipment is not lubricated and kept free from oil and grease.
open the cylinder valve slowly and check for any leaks.
Leaks
Having connected the regulator or manifold yoke to the cylinder check the connections for leaks using the following procedure:
• Should leaks occur this will usually be evident by a hissing noise.
• Should a leak occur between the valve outlet and the regulator or manifold yoke, depressurise and remove the fitting and fit an approved sealing washer. Reconnect the fitting to the valve with moderate force only, fitting a replacement regulator or manifold tailpipe as required.
• Sealing or jointing compounds must never be used to cure a leak.
• If leak persists, label cylinder and return to BOC
Use of Cylinders
When Medical Carbon Dioxide cylinders are in use ensure that they are:
• only used for medicinal purposes.
• turned off, when not in use, using only moderate force to close the valve
• only moved with the appropriate size and type of trolley or handling device.
• handled with care and not knocked violently or allowed to fall.
• firmly secured to a suitable cylinder support when in use.
• not allowed to have any markings, labels or batch labels obscured or removed
• not used in the vicinity of persons smoking or near naked lights.
After use
When the Medical Carbon Dioxide cylinders are empty ensure that the:
• cylinder valves is closed using moderate force only and the pressure in the regulator or tailpipe released.
• valve outlet cap, where fitted, is replaced
• empty cylinders are immediately returned to an empty cylinder storage area for return to BOC.
ADMINISTRATIVE DATA
7. MARKETING AUTHORISATION HOLDER BOC Ltd.
The Priestley Centre 10 Priestley Road The Surrey Research Park GUILDFORD Surrey, GU2 5XY.
MARKETING AUTHORISATION NUMBER
8.
PL 0735/5006R
9. DATE OF FIRST AUTHORISATION/RENEWAL OF
AUTHORISATION
|
Date First Granted: |
01/09/1972 |
|
Date of Renewal |
23/01/1991 |
10 DATE OF REVISION OF THE TEXT
25/05/2016