Medical Nitrous Oxide
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Medical Nitrous Oxide.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Medical Nitrous Oxide cylinders are supplied to the following specification: Medical Nitrous Oxide Purity 98.0% v/v
The Medical Nitrous Oxide cylinder specification complies with the current European Pharmacopeia (0416)
3 PHARMACEUTICAL FORM
Medicinal Gas, Compressed
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Nitrous oxide is used:
• when an inhalation anaesthetic is required, the administration of Nitrous Oxide is usually accompanied by simultaneous administration of a volatile agent such as halothane, ethrane, etc.
• in the relief of severe pain, usually in emergency situations, by inhalation with 50% oxygen
• in short-term procedures which inevitably involve pain, such as wound and burn dressing, wound debridement and suturing. Administered usually with 50% oxygen.
• in dental work to provide short-term analgesia for tooth extraction and other brief procedure, administered with 50% oxygen
• occasionally as an insufflating agent in laparoscopy
• in cryosurgery as a refrigerant
4.2 Posology and method of administration
Nitrous Oxide is administered through a face mask or tracheal tube by means of an anaesthetic apparatus. The gas is breathed in by the patient and absorbed through the lungs.
Where the clinical indication is the production of general anaesthesia, it should he noted that:
• in the average adult, Nitrous Oxide is administered by inhalation through a suitable anaesthetic apparatus in concentrations up to 80% with oxygen as the balance
• as people age, there is a steady reduction in the indices of cardiac and respiratory function evinced by a lowering of cardiac output and in lung ventilation and perfusion. In addition, there is an increase in dead space in the lung which increases minute ventilation. Cerebral blood flow is reduced by up to 30%. The result of these changes means that susceptibility to anaesthesia is increased. Nitrous Oxide is, therefore, more useful in the elderly and the depressant effects of added agents are reduced
• there are no essential differences in clinical indications between the adult and child
• Nitrous Oxide is strongly recommended in the anaesthesia of neonates
• in obstetrical anaesthesia, the nitrous oxide level is kept below 70% to allow a substantial oxygen level to be provided. Nitrous Oxide plays a major role because injected agents depress the breathing of the infant and volatile agents depress uterine contraction
• As a general rule, the more ill the patient, the more susceptible is the patient to other anaesthetic agents and the more Nitrous Oxide is relied upon.
Nitrous Oxide should not be used for more than a total of 24 hours, or more frequently than every 4 days, without close clinical supervision and haematological monitoring (see sections 4.4 and 4.8).
Nitrous oxide is usually not sufficient to create an adequate anaesthetic effect on its own, and should therefore be used in combination with appropriate doses of another anaesthetic when used for general anaesthesia. Nitrous oxide has additive interaction with most other anaesthetics (see interactions 4.5).
4.3 Contraindications
Nitrous Oxide should not be used with any condition where gas is entrapped within a body and where its expansion might he dangerous, such as:
• head injuries with impairment of consciousness
• artificial, traumatic or spontaneous pneumothorax
• air embolism
• decompression sickness
• following a recent underwater dive
• following air encephalography
• severe bullous emphysema
• during myringoplasty
• gross abdominal distension
• intoxication
• maxillofacial injuries
• in patients having received recent intraocular injection of gas (such as SF6)
4.4 Special warnings and precautions for use
Nitrous oxide causes inactivation of vitamin B12, which is a co-factor of methionine synthase. Folate metabolism is consequently interfered with and DNA synthesis is impaired following prolonged administration of Nitrous Oxide. Prolonged or frequent use of Nitrous Oxide may result in megaloblastic marrow changes, myeloneuropathy and sub acute combined degeneration of the spinal cord.
Nitrous Oxide should not be used for more than a total of 24 hours, or more frequently than every 4 days, without close clinical supervision and haematological monitoring. Specialist advice should be sought from a haematologist in such cases. Haematological assessment should include an assessment for megaloblastic change in red cells and hypersegmentation of neutrophils. Neurological toxicity can occur without anaemia or macrocytosis and with B12 levels in the normal range.
In patients with undiagnosed subclinical deficiency of vitamin B12, neurological toxicity has occurred after single exposures to Nitrous Oxide during general anaesthesia.
Assessment of vitamin B12 levels should be considered in people with risk factors for vitamin B12 deficiency prior to using Nitrous Oxide anaesthesia. Risk factors include the elderly, those with poor or vegetarian diet, and previous history of anaemia.
Nitrous Oxide should never be given with less than 21% oxygen, but a maximum of 30% oxygen should be used during anaesthesia (except when used in combination with a volatile anaesthetic agent) and more at altitude and in the presence of disorders affecting oxygenation.
Reduced fertility in healthcare personnel has been reported where they have been repeatedly exposed to high levels of Nitrous Oxide above the specified occupational exposure limits in inadequately ventilated rooms. There is no documented evidence to confirm or exclude the existence of any causal connection between these cases and exposure to Nitrous Oxide.
Scavenging of waste Nitrous Oxide gas should be used to reduce operating theatre and equivalent treatment room levels to a level below 100 ppm of ambient Nitrous Oxide.
In patients taking other centrally acting medicinal products, such as morphine derivatives and/or benzodiazepines, concomitant administration of Nitrous Oxide may result in increased sedation, and consequently have effects on respiration, circulation and protective reflexes. If Nitrous Oxide is to be used in such patients, this should take place under the supervision of appropriately trained personnel (see Section 4.5).
At the end of a Nitrous Oxide/Oxygen anaesthesia, withdrawal of the mask leads to an outpouring of Nitrous Oxide from the lung and consequent dilution of oxygen in incoming air. This results in "diffusion hypoxia" and is counteracted by giving 100% oxygen for a few minutes when the flow of Nitrous Oxide is stopped.
Nitrous Oxide is non flammable but strongly supports combustion and should not be used near sources of ignition.
Smoking should be prohibited when using Nitrous Oxide
Under no circumstances should oils or grease be used to lubricate any part of the Nitrous Oxide cylinder or the associated equipment used to deliver the gas to the patient.
Where moisturising preparations are required for use with a facemask, oil based creams should not be used.
Check that hands are clean and free from any oils or grease.
Where alcohol gels are used to control microbiological cross-contamination ensure that all alcohol has evaporated before handling Nitrous Oxide cylinders or equipment
Nitrous Oxide is stored in high pressure gas cylinders as a liquid under pressure. Rapid opening of the valve can cause the discharged gas to re-liquefy. This liquid can cause cold bums if in contact with the skin. Cylinders should only be used in the vertical position with the valve uppermost. If not, liquid may be discharged when the valve is opened.
4.5 Interaction with other medicinal products and other forms of interaction
Nitrous Oxide inactivates vitamin B12 and potentiates the effects of methotrexate on folate metabolism.
There are additive effects when Nitrous Oxide is used in combination with other inhaled anaesthetics or drugs having a central depressant action (e.g. opiates, benzodiazepines and other psychotropics). These interactions have clear effects in clinical practise, decreasing the dose needed for the other agents combined with nitrous oxide, causing less cardiovascular and respiratory depression and increasing speed of emergence.
4.6 Pregnancy and lactation
Pregnancy
Mild skeletal teratogenic changes have been observed on pregnant rat embryos when the dam has been exposed to high concentrations of nitrous oxide during the period of organogenesis.
However no increased incidence of foetal malformation has been discovered in 8 epidemiological studies and case reports in human beings.
There is no published material which shows that nitrous oxide is toxic to the human foetus.
Therefore, there is no absolute contraindication to its use in the first 16 weeks of pregnancy.
There are no known adverse effects to using Nitrous Oxide during the breast-feeding period.
4.7. Effects on Ability to Drive and use Machines
Nitrous Oxide is rapidly eliminated but driving, use of machinery and other psycho-motor activities should not be undertaken until 12 hours have elapsed after Nitrous Oxide anaesthesia.
4.8 Undesirable effects
Events such as euphoria, disorientation, sedation, nausea, vomiting, dizziness and generalised tingling are commonly described. These events are generally minor and rapidly reversible.
Prolonged or frequent use of Nitrous Oxide, including heavy occupational exposure and addiction, may result in megaloblastic anaemia. Agranulocytosis has been reported following prolonged nitrous oxide administration (see section 4.4).
Myeloneuropathy and sub acute combined degeneration have also been reported following prolonged or frequent use. However in patients with undiagnosed subclinical deficiency of vitamin B12, neurological toxicity has occurred after a single exposure to Nitrous Oxide for anaesthesia (see section 4.4).
Addiction may occur
Nitrous oxide passes into all gas containing spaces in the body faster than nitrogen passes out. Prolonged exposure may result in bowel distension, middle ear damage and rupture of ear drums.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via The Yellow Card System www.mhra.gov.uk/yellowcard
4.9. Overdose
Inappropriate, unwitting or deliberate inhalation of Nitrous Oxide will ultimately result in unconsciousness, passing through stages of increasing light-headedness and intoxication, and, if the victim were to be within a confined space, death from anoxia could result. The treatment is removal to fresh air, mouth-to-mouth resuscitation and, if necessary, the use of an oxygen resuscitator.
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group - general anaesthetics ATC Code - N01AX13
The characteristics of Nitrous Oxide are:
• sweet smelling, colourless gas
• molecular weight 44.01
• boiling point -88.6oC (at 1 bar)
• density 1.875 kg/m3 (at 15oC)
Nitrous Oxide is not very soluble in water but is fifteen times more soluble than oxygen. Water dissolves Nitrous Oxide, taking 100 vol %, and blood plasma 45 vol %.
Nitrous Oxide is eliminated unchanged from the body mostly by the lungs. Nitrous Oxide is a potent analgesic and a weak anaesthetic. Induction with Nitrous Oxide is relatively rapid, but a concentration of about 70% is needed to produce unconsciousness.
Endorphins are probably involved in the analgesic effect; a concentration of 25% Nitrous Oxide is usually adequate to provide a marked reduction in pain.
5.2. Pharmacokinetic properties
Nitrous Oxide is a low potency inhalation anaesthetic and only slightly soluble. The advantage of this is that concentrations not greater than 70% are used and induction of anaesthesia and recovery occur quickly.
At a constant inspired concentration, the rise time of alveolar concentrations is faster than that of any other anaesthetic agent. The elimination of Nitrous Oxide is faster than that of any other anaesthetic.
The blood/gas partition coefficient of nitrous oxide at 37°C is 0.46 compared with that of nitrogen of 0.015, causing Nitrous Oxide to expand into internal gas spaces.
Under normal anaesthesia, the adult body contains about 25 litres of gaseous Nitrous Oxide (this gives some notion of its essential safety and lack of acute toxicity).
The flow of Nitrous Oxide out from the tissues through the lungs at the end of anaesthesia may lead to a degree of transient hypoxia.
Preclinical safety data
5.3
The current published toxico-pharmacological data indicates that Medical Nitrous Oxide will not be harmful to humans.
6. PHARMACEUTICAL PARTICULARS 6.1. List of excipients
Nitrogen
6.2 Incompatibilities
Nitrous Oxide is chemically inactive and will not react with other compounds at normal temperatures.
Medical Nitrous Oxide strongly supports combustion and will cause substances to burn vigorously, including some materials that do not normally burn in air. It is highly dangerous in the presence of oils, greases, tarry substances and many plastics due to the risk of spontaneous combustion in the presence of Nitrous Oxide in relatively high concentrations.
6.3. Shelf life
36 months
6.4 Special precautions for storage
Medical Nitrous Oxide cylinders should be:
• Stored under cover, preferably inside, kept dry and clean, not subjected to extremes of heat or cold and stored away from stocks of material
• Not stored near stocks of combustible materials or near sources of heat.
• Stored separately from industrial and other non-medical cylinders.
• Stored to maintain separation between full and empty cylinders.
• Used in strict rotation so that cylinders with the earliest filling date are used first.
• Stored separately from other medical cylinders within the store
• F size cylinders and larger should be stored vertically. E size cylinders and smaller should be stored horizontally.
Warning notices prohibiting smoking and naked lights must be posted clearly in the cylinder storage area and the Emergency Services should be advised of the location of the cylinder store.
Precautions should be taken to protect the cylinders from theft.
Care is needed when handling and using Medical Nitrous Oxide cylinders
6.5 Nature and contents of container
A summary of Medical Nitrous Oxide cylinders, their size and construction, type of valve fitted is detailed below:
|
Cylinder Size |
Gas Content (litres) |
Cylinder Water Capacity (litres) |
Cylinder Construction |
Outlet Connection |
Valve Outlet Pressure bar(g) |
|
D |
900 |
2.3 |
Steel |
Pin Index (ISO 407) |
44 |
|
E |
1 800 |
5. |
Steel |
Pin Index (ISO 407) |
44 |
|
F |
3 600 |
9.43 |
Steel |
11/16" x 20 TPI (M) (BS 341(Type 8)) |
44 |
|
G |
9 000 |
23.6 |
Steel |
11/16" x 20 TPI (M) (BS 341(Type 8)) |
44 |
|
J |
18 000 |
47.2 |
Steel |
11/16" x 20 TPI (M) (BS 341(Type 8)) |
44 |
Cylinders
All cylinders used for the storage of Medical Nitrous Oxide are manufactured from high tensile steel designed with working pressure of at least 137 bar(g).
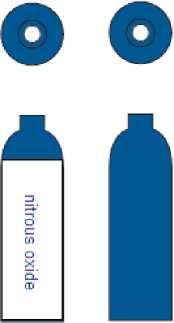
The colour coding of the shoulders of Medical Nitrous Oxide cylinders is blue (RAL 5010).
The colour coding of the cylinder body is white (RAL 9010). Cylinders also carry the nitrous oxide name on the body of the cylinder.
For a limited period, cylinders may have blue bodies. These cylinders do not have the name nitrous oxide on the body of the cylinder.
The programme to convert all Medical Nitrous Oxide cylinders to white bodies will be completed by 2025.
Cylinder Valves
Medical Nitrous Oxide cylinders are supplied with two main types of cylinder valves.
D and E size cylinders are fitted with valves with outlet connections that conform to ISO 407 (pin index) and F, G and J size cylinders are fitted with outlet connections that conform to BS 341 (Type 8) (11/16" x 20 TPI (M)).
All cylinder valves are constructed from high tensile brass with a steel spindle fitted with a Nylon 6.6 insert.
6.6 Special precautions for disposal and other handling
All personnel handling Medical Nitrous Oxide gas cylinders should have adequate knowledge of:
• properties of the gas
• correct operating procedures for the cylinder
• precautions and actions to be taken in the event of an emergency.
Preparation for Use
To prepare the cylinder for use:
• remove the tamper evident seal and the valve outlet protection. Ensure the cap is retained so that it can be refitted after use. Do not remove and discard batch labels fitted to the cylinder
• ensure that an appropriate Medical Nitrous Oxide regulator is selected for connection to the cylinder.
• ensure the connecting face on the regulator is clean and the sealing washer fitted is in good condition.
• connect the regulator, using moderate force only and connect the tubing to the regulator / flowmeter outlet. Only the appropriate regulator should be used for the particular gas concerned.
• open the cylinder valve slowly and check for any leaks
Leaks
Having connected the regulator or manifold yoke to the cylinder check the
connections for leaks using the following procedure:
• should leaks occur this will usually be evident by a hissing noise.
• should a leak occur between the valve outlet and the regulator or manifold yoke, depressurise and remove the fitting and fit an approved sealing washer. Reconnect the fitting to the valve with moderate force only, fitting a replacement regulator or manifold tailpipe as required.
• sealing or jointing compounds must never be used to cure a leak.
• never use excessive force when connecting equipment to cylinders.
• if leak persists. label cylinder and return to BOC
Use of Cylinders
When Medical Nitrous Oxide cylinders are in use ensure that they are:
• only used for medicinal purposes.
• turned off, when not in use, using moderate force to close the valve
• only moved with the appropriate size and type of trolley or handling device.
• handled with care and not knocked violently or allowed to fall.
• firmly secured to a suitable cylinder support when in use.
• not allowed to have any markings, labels or batch labels obscured or removed.
• not used in the vicinity of persons smoking or near naked lights.
• used vertically with the valve uppermost
• used in a well ventilated area with waste gas scavenging systems in place to maintain the average occupational exposure level of the healthcare professional to less than 100ppm (over an 8 hour period).
When the Medical Nitrous Oxide cylinder is empty ensure that:
• the cylinder valve is closed using moderate force only and the pressure in the regulator or tailpipe released.
• the valve outlet cap, where fitted, is replaced
• the empty cylinders are immediately returned to the empty cylinder store for return to BOC.
7 MARKETING AUTHORISATION HOLDER
BOC Ltd
The Priestley Centre 10 Priestly Rd Surrey Research Park
Guildford GU2 5XY
8. MARKETING AUTHORISATION NUMBER
PL 00735/5001R
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
18 January 1991
10 DATE OF REVISION OF THE TEXT
25/05/2016