Memantine Hydrochloride Teva 10Mg/Ml Oral Solution
Package leaflet: Information for the patient
Memantine hydrochloride 10 mg/ml Oral Solution
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Memantine hydrochloride is and what it is used for
2. What you need to know before you take Memantine hydrochloride
3. How to take Memantine hydrochloride
4. Possible side effects
5. How to store Memantine hydrochloride
6. Contents of the pack and other information
1. What Memantine hydrochloride is and what it is used for How does Memantine hydrochloride work
Memantine hydrochloride belongs to a group of medicines known as anti-dementia medicines. Memory loss in Alzheimer’s disease is due to a disturbance of message signals in the brain. The brain contains so-called N-methyl-D-aspartate (NMDA)-receptors that are involved in transmitting nerve signals important in learning and memory. Memantine hydrochloride belongs to a group of medicines called NMDA-receptor antagonists. Memantine hydrochloride acts on these NMDA-receptors improving the transmission of nerve signals and the memory.
What is Memantine hydrochloride used for
Memantine hydrochloride is used for the treatment of patients with moderate to severe Alzheimer’s disease.
2. What you need to know before you take Memantine hydrochloride Do not take Memantine hydrochloride
• if you are allergic to memantine hydrochloride or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before taking Memantine hydrochloride.
• if you have a history of epileptic seizures.
• if you have recently experienced a myocardial infarction (heart attack), or if you are suffering from congestive heart failure or from an uncontrolled hypertension (high blood pressure).
In these situations the treatment should be carefully supervised, and the clinical benefit of Memantine hydrochloride reassessed by your doctor on a regular basis.
You should inform your doctor if you have recently changed or intend to change your diet substantially (e.g. from normal diet to strict vegetarian diet) or if you are suffering from states of renal
tubulary acidosis (RTA, an excess of acid-forming substances in the blood due to renal dysfunction (poor kidney function)) or severe infections of the urinary tract (structure that carries urine), as your doctor may need to adjust the dose of your medicine.
If you suffer from renal impairment (kidney problems), your doctor should closely monitor your kidney function and if necessary adapt the memantine doses accordingly.
The use of medicines called
• amantadine (for the treatment of Parkinson’s disease),
• ketamine (a substance generally used as an anaesthetic),
• dextromethorphan (generally used to treat cough) and
• other NMDA-antagonists
at the same time should be avoided.
Children and adolescents
Memantine hydrochloride is not recommended for children and adolescents under the age of 18 years. Other medicines and Memantine hydrochloride
Tell your doctor or pharmacist if you are taking/using, have recently taken/used or might take/use any other medicines.
In particular, Memantine hydrochloride may change the effects of the following medicines and their dose may need to be adjusted by your doctor:
• amantadine, ketamine, dextromethorphan
• dantrolene, baclofen
• cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine
• hydrochlorothiazide (or any combination with hydrochlorothiazide)
• anticholinergics (substances generally used to treat movement disorders or intestinal cramps)
• anticonvulsants (substances used to prevent and relieve seizures)
• barbiturates (substances generally used to induce sleep)
• dopaminergic agonists (substances such as L-dopa, bromocriptine)
• neuroleptics (substances used in the treatment of mental disorders)
• oral anticoagulants
If you go into hospital, let your doctor know that you are taking Memantine hydrochloride. Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
The use of memantine in pregnant women is not recommended.
Women taking Memantine hydrochloride should not breast-feed.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive and to use machines safely.
Also, Memantine hydrochloride may change your reactivity, making driving or operating machinery inappropriate.
Memantine hydrochloride contains sorbitol
This medicine contains sorbitol. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine. Your doctor will advise you.
3. How to take Memantine hydrochloride
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Dosage
The recommended dose of Memantine hydrochloride for adults and elderly patients is four pump actuations or 2 ml solution, equivalent to 20 mg once a day. In order to reduce the risk of side effects this dose is achieved gradually by the following daily treatment scheme.
• Pump
One pump actuation (0.5 ml solution) contains 5 mg memantine hydrochloride.
|
week 1 |
one pump actuation, equivalent to 0.5 ml solution (5 mg) |
|
week 2 |
two pump actuations, equivalent to 1 ml solution (10 mg) |
|
week 3 |
three pump actuations, equivalent to 1.5 ml solution (15 mg) |
|
week 4 and beyond |
four pump actuations, equivalent to 2 ml solution (20 mg) |
The usual starting dose is one pump actuation once daily (1 x 5 mg) for the first week. This dose is increased in the second week to two pump actuations once daily (1 x 10 mg), and in the third week to three pump actuations once daily (1 x 15 mg). From the fourth week the recommended dose is four pump actuations once daily (1x 20 mg).
• Dosing Pipette
0.5 ml solution contains 5 mg memantine hydrochloride.
|
week 1 |
0.5 ml solution (5 mg) |
|
week 2 |
1 ml solution (10 mg) |
|
week 3 |
1.5 ml solution (15 mg) |
|
week 4 and beyond |
2 ml solution (20 mg) |
The usual starting dose is 0.5 ml solution once daily (1 x 5 mg) for the first week. This dose is increased in the second week to 1 ml solution once daily (1 x 10 mg), and in the third week to 1.5 ml solution once daily (1 x 15 mg). From the fourth week the recommended dose is 2 ml solution once daily (1x 20 mg).
Dosage in patients with impaired kidney function
If you have impaired kidney function, your doctor will decide upon a dose that suits your condition. In this case, monitoring of your kidney function should be performed by your doctor at specified intervals.
Method of administration
Memantine hydrochloride should be administered orally once a day. To benefit from your medicine you should take it regularly every day at the same time of the day.
The solution can be taken with or without food.
The solution must not be poured, pumped or pipetted directly into the mouth from the bottle or pump/dosing pipette. Measure the dose onto a spoon or into a glass of water, using the pump or the dosing pipette.
For detailed instructions on the preparation and handling of the medicine see end of this leaflet. Duration of treatment
Continue to take Memantine hydrochloride as long as it is of benefit to you. Your doctor should assess your treatment on a regular basis.
If you take more Memantine hydrochloride than you should
• In general, taking too much Memantine hydrochloride should not result in any harm to you. You may experience increased symptoms as described in section 4. “Possible side effects”.
• If you take a large overdose of Memantine hydrochloride, contact your doctor or get medical advice, as you may need medical attention.
If you forget to take Memantine hydrochloride
• If you find you have forgotten to take your dose of Memantine hydrochloride, wait and take your next dose at the usual time.
• Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In general, the observed side effects are mild to moderate.
Common (may affect up to 1 in 10 people):
• Headache, sleepiness, constipation, elevated liver function test, dizziness, balance disorder, shortness of breath, high blood pressure and drug hypersensitivity
Uncommon (may affect up to 1 in 100 people):
• Tiredness, fungal infections, confusion, hallucinations, vomiting, abnormal gait, heart failure and venous blood clotting (thrombosis/thromboembolism)
Very Rare (may affect up to 1 in 10,000 people):
• Seizures
Not known (frequency cannot be estimated from the available data):
• Inflammation of the pancreas, inflammation of the liver (hepatitis) and psychotic reactions
Alzheimer’s disease has been associated with depression, suicidal ideation and suicide. These events have been reported in patients treated with memantine.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Memantine hydrochloride Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and the bottle after EXP. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Shelf life after first opening: 12 weeks.
Pump
The bottle with the mounted pump must be kept and transported in an upright position only.
Do not throw away any medicines via wastewater <or household waste>. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Memantine hydrochloride contains
The active substance is memantine hydrochloride.
Each ml of oral solution contains 10 mg of memantine hydrochoride equivalent to 8.31 mg memantine.
Pump
Each pump actuation delivers 0.5 ml solution containing 5 mg memantine hydrochloride equivalent to 4.16 mg memantine.
Dosing Pipette
0.5 ml solution contains 5 mg memantine hydrochloride equivalent to 4.16 mg memantine.
The other ingredients are:
Sorbitol liquid 70% (non-crystallising) (E 420), potassium sorbate, purified water.
What Memantine hydrochloride looks like and contents of the pack
Clear colourless to almost colourless oral solution.
Packs of 50 ml, 100 ml and 10 x 50 ml oral solution.
Each pack contains either a pump packed in a plastic bag or a dosing pipette.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Teva UK Limited, Eastbourne, BN22 9AG, UK
Manufacturer
Merckle GmbH, Ludwig-Merckle-Str. 3, 89143 Blaubeuren, Germany OR*
TEVA Pharmaceutical Works Private Limited Company, Pallagi ut 13, 4042 Debrecen, Hungary OR*
TEVA UK Limited, Eastbourne, BN22 9AG, UK OR*
TEVA Sante, Rue Bellocier, 89100 Sens, France OR*
Teva Pharma B.V., Swensweg 5, 2031 GA Haarlem,The Netherlands * Only the actual site of batch release will appear on the printed version of the leaflet This leaflet was last revised in 06/2015 PL 00289/1755
• Instruction for proper use of the pump

Mounting the dosing pump on the bottle
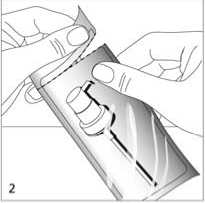
Take the dosing pump out of the plastic bag (fig. 2) and place it on top of the bottle. Slide the plastic dip tube carefully into the bottle.
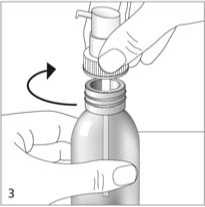
Hold the dosing pump onto the neck of the bottle and screw it clockwise until it fits firmly (fig. 3). The dosing pump is only screwed on once when starting the use, and should never be unscrewed.
Preparing the dosing pump
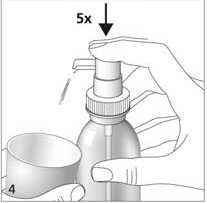
When used for the first time, the dosing pump does not dispense the correct amount of oral solution.
Therefore, the pump must be prepared (primed) by pushing the dosing pump head down completely five times in succession (fig. 4). The solution thus dispensed is discarded.
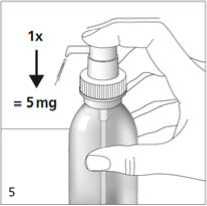
The next time the dosing pump head is pushed downwards completely (equivalent to one pump actuation), it dispenses the correct dose (fig. 5).
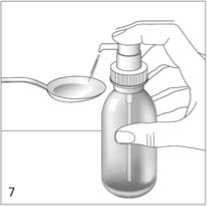
Correct use of the dosing
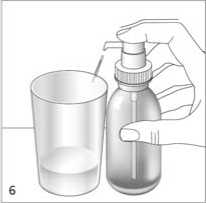
Place the bottle on a flat, horizontal surface, for example a table top, and only use it in an upright position. Hold a glass with a little water or a spoon below the nozzle. Push down the dosing pump head in a firm but calm and steady manner - not too slowly (fig. 6, fig. 7).
The dosing pump head can then be released and is ready for the next pump actuation.
If the pump does not function properly, consult your doctor or a pharmacist.
• Instruction for proper use of the dosing pipette
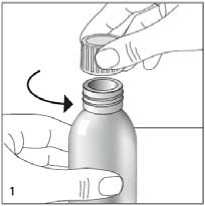
Unscrew the cap fully by turning anticlockwise (fig 1).
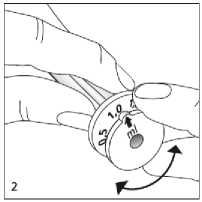
Before using the dosing pipette, set the required dosage volume (ml) first. The marks indicating the dosage volume can be found on the top of the bottom ring. Turn the piston by the top ring until the arrow points to the required dosage volume and also clicks in slightly (the piston must be fully depressed while doing this) (fig 2).
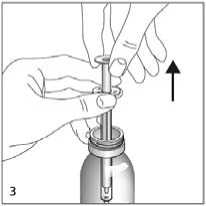
Insert the preset dosing pipette into the bottle. Hold the bottom ring firmly and pull the piston up by the top ring as far as it will go.
Hold the dosing pipette by the bottom ring and remove from the bottle (fig 3).
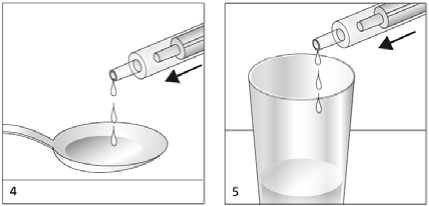
Dispense the solution in the dosing pipette onto a spoon or into a glass of water by pressing the piston in (fig 4, fig 5).
Then seal the bottle again tightly with the cap.