Memantine Sandoz 10 Mg/Ml Oral Solution
SZ00000LT000
PACKAGE LEAFLET: INFORMATION FOR THE USER
Memantine hydrochloride
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Memantine Oral Solution is and what it is used for
2. What you need to know before you take Memantine Oral Solution
3. How to take Memantine Oral Solution
4. Possible side effects
5. How to store Memantine Oral Solution
6. Contents of the pack and other information
How does Memantine Oral Solution work
Memantine Oral Solution belongs to a group of medicines known as anti-dementia medicines.
Memory loss in Alzheimer’s disease is due to a disturbance of message signals in the brain. The brain contains so-called N-methyl-D-aspartate (NMDA)-receptors that are involved in transmitting nerve signals important in learning and memory. Memantine Oral Solution belongs to a group of medicines called NMDA-receptor antagonists. Memantine Oral Solution acts on these NMDA-receptors improving the transmission of nerve signals and the memory.
What is Memantine Oral Solution used for
Memantine Oral Solution is used for the treatment of patients with moderate to severe Alzheimer’s disease.
to strict vegetarian diet) or if you are suffering from states of renal tubulary acidosis (RTA, an excess of acid-forming substances in the blood due to renal dysfunction (poor kidney function)) or severe infections of the urinary tract (structure that carries urine), as your doctor may need to adjust the dose of your medicine.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
The use of Memantine Oral Solution in pregnant women is not recommended.
Women taking Memantine Oral Solution should not breast-feed.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive and to use machines safely. Also, Memantine Oral Solution may change your reactivity, making driving or operating machinery inappropriate.
Memantine Oral Solution contains sorbitol (E420).
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
Do not take Memantine Oral Solution
• if you are allergic to memantine hydrochloride or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before taking Memantine Oral Solution:
• if you have a history of epileptic seizures
• if you have recently experienced a myocardial infarction (heart attack), or if you are suffering from congestive heart failure or from an uncontrolled hypertension (high blood pressure)
In these situations the treatment should be carefully supervised, and the clinical benefit of Memantine Oral Solution reassessed by your doctor on a regular basis.
If you suffer from renal impairment (kidney problems), your doctor should closely monitor your kidney function and if necessary adapt the memantine doses accordingly.
The use of medicinal products called amantadine (for the treatment of Parkinson's disease), ketamine (a substance generally used as an anaesthetic), dextromethorphan (generally used to treat cough) and other NMDA-antagonists at the same time should be avoided.
Children and adolescents
Memantine Oral Solution is not recommended for children and adolescents under the age of 18 years.
Other medicines and Memantine Oral Solution
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
In particular, Memantine Oral Solution may change the effects of the following medicines and their dose may need to be adjusted by your doctor:
• amantadine, ketamine, dextromethorphan
• dantrolene, baclofen
• cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine
• hydrochlorothiazide (or any combination with hydrochlorothiazide)
• anticholinergics (substances generally used to treat movement disorders or intestinal cramps)
• anticonvulsants (substances used to prevent and relieve seizures)
• barbiturates (substances generally used to induce sleep)
• dopaminergic agonists (substances such as L-dopa, bromocriptine)
• neuroleptics (substances used in the treatment of mental disorders)
• oral anticoagulants
If you go into hospital, let your doctor know that you are taking Memantine Oral Solution.
Memantine Oral Solution with food and drink
You should inform your doctor if you have recently changed or intend to change your diet substantially (e.g. from normal diet
Always take Memantine Oral Solution exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Dosage
The recommended dose for adults and elderly patients is 20 mg once a day.
In order to reduce the risk of side effects this dose is achieved gradually by the following daily treatment scheme:
|
Period of intake |
Dosage once daily |
|
week 1 |
0.5 ml |
|
week 2 |
1 ml |
|
week 3 |
1.5 ml |
|
week 4 and beyond |
2 ml |
Dosage in patients with impaired kidney function
If you have impaired kidney function, your doctor will decide upon a dose that suits your condition. In this case, monitoring of your kidney function should be performed by your doctor at specified intervals.
Administration
Memantine Oral Solution should be administered orally once a day. To benefit from your medicine you should take it regularly every day at the same time of the day.
Instructions for use
Read these instructions carefully so that you know how to use this medicine.
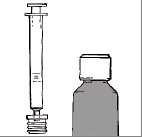
The medicine-kit consists of three parts:
• a bottle containing solution
• a plastic adapter, connected to
• a 2 ml oral syringe, with 0.5 ml graduations
Continued on the next page >>
|
Artwork Proof Box Ref: Licence application | ||
|
Proof no. |
Date prepared: |
Font size: |
|
001.0 |
24/05/2013 |
8pt |
|
Colours: | Black |
□ |
Fonts: Helvetica |
|
□ |
□ | |
|
^ Dimensions: |
1 80 x 420 mm |
_y |
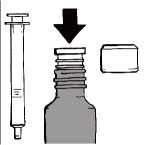
Before the first use
Hold the open bottle upright on a table. Remove the plastic adapter from the oral syringe and push it firmly into the neck of the bottle, as far as you can. The adapter must be kept in the bottle until the last dose is withdrawn. To dispense a dose, please follow all instructions in "Preparing a dose" up to "Cleaning".
Preparing a dose
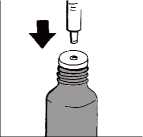
Turn the cap to open the bottle. Check the plunger is fully down inside the barrel of the oral syringe.
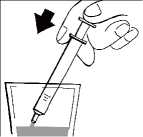
Keep the bottle upright and insert the oral syringe firmly into the plastic adapter, as shown in the picture.
Uncommon, may affect up to 1 in 100 people:
• tiredness, fungal infections, confusion, hallucinations, vomiting, abnormal gait, heart failure and venous blood clotting (thrombosis/thromboembolism)
Very Rare, may affect up to 1 in 10,000 people:
• seizures
Not known, frequency cannot be estimated from the available data:
• inflammation of the pancreas, inflammation of the liver (hepatitis) and psychotic reactions
Alzheimer's disease has been associated with depression, suicidal ideation and suicide. These events have been reported in patients treated with this medicine.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (www.mhra.gov.uk/yellowcard). By reporting side effects you can help provide more information on the safety of this medicine.
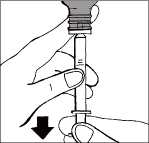
Withdrawing the dose
Hold the oral syringe in place and carefully turn the bottle upside down. Slowly pull the plunger of the syringe down fully so that the oral syringe fills with solution. Push the plunger back up completely to expel any large air bubbles that may be trapped inside the oral syringe.
Slowly pull the plunger down until the barrel ring reaches the mark corresponding to the number of milliliters or milligrams you need to take (0.5 ml = 5 mg, 1 ml = 10 mg,
1.5 ml = 15 mg, 2 ml = 20 mg).
Keep this medicine out of the sight and reach of children.
This medicinal product does not require any special storage conditions.
Do not use this medicine after the expiry date which is stated on the carton and the bottle label after “EXP". The expiry date refers to the last day of that month.
Once opened, the contents of the bottle should be used within 6 months.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Carefully turn the bottle the right way up. Disconnect the oral syringe by gently twisting it out of the plastic adapter.
Administering the dose
The solution can be swallowed directly from the oral syringe. The patient must be sitting upright and the plunger must be pushed slowly to allow the patient to swallow.
Alternatively, the dose can be mixed in a small glass of water just prior to administration. Stir and drink the entire mixture right away.
The oral solution can be taken with or without food.
Replace the cap after use, leaving the adapter in place.
Cleaning
After use, wipe the outside of the oral syringe with a dry, clean tissue.
Duration of treatment
Continue to take Memantine Oral Solution as long as it is of benefit to you. Your doctor should assess your treatment on a regular basis.
If you take more Memantine Oral Solution than you should
• In general, taking too much Memantine Oral Solution should not result in any harm to you. You may experience increased symptoms as described in section 4. "Possible side effects“.
• If you take a large overdose of Memantine Oral Solution, contact your doctor or get medical advice, as you may need medical attention.
If you forget to take Memantine Oral Solution
• If you find you have forgotten to take your dose of Memantine Oral Solution, wait and take your next dose at the usual time.
• Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In general, the observed side effects are mild to moderate.
Common, may affect up to 1 in 10 people:
• headache, sleepiness, constipation, elevated liver function tests, dizziness, balance disorders, shortness of breath, high blood pressure and drug hypersensitivity
What Memantine Oral Solution contains
The active substance is memantine.
Each ml of memantine oral solution contains 10 mg memantine hydrochloride which is equivalent to 8.31 mg memantine.
The other ingredients are potassium sorbate, sorbitol liquid (non crystallising) (E420), sodium hydroxide (for pH adjustment), hydrochloride acid (for pH adjustment) and purified water.
What Memantine Oral Solution looks like and contents of the pack
Memantine 10 mg/ml Oral Solution is a colourless and clear solution.
This medicine is available in amber glass bottles type III containing 20 ml, 50 ml or 100 ml of oral solution. The bottles are closed with HDPE screw cap with tamper-evident ring and packed in a carton box together with an oral syringe (LDPE and PS) connected to a press-in bottle adapter (LDPE). The oral syringe has a main graduation in steps of 0.5 ml and 5 mg (0.5, 1, 1.5 2 ml respectively 5, 10, 15, 20 mg) and a fine graduation in steps of 0.1 ml (= 1 mg).
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing authorization holder:
Sandoz Ltd,
Frimley Business Park, Frimley,
Camberley, Surrey, GU16 7SR,
United Kingdom.
Manufacturer:
Lek Pharmaceuticals d.d.,
Verovskova 57, 1526 Ljubljana, Slovenia
or
LEK S.A.,
ul. Domaniewska 50 C,
02-672 Warszawa, Poland
or
Salutas Pharma GmbH,
Otto-von-Guericke-Allee 1,
39179 Barleben, Germany
or
S.C. Sandoz, S.R.L.,
Str. Livezeni nr. 7A,
RO-540472 Targu-Mures, Romania
or
Weimer Pharma GmbH,
Im Steingerust 30, 76437 Rastatt, Germany.
This leaflet was last revised in 05/2013.
SZ00000LT000
|
Artwork Proof Box Ref: Licence application | ||
|
Proof no. |
Date prepared: |
Font size: |
|
001.0 |
24/05/2013 |
8pt |
|
Colours: | Black |
□ |
Fonts: Helvetica |
|
□ |
□ | |
|
^ Dimensions: |
1 80 x 420 mm |
_y |