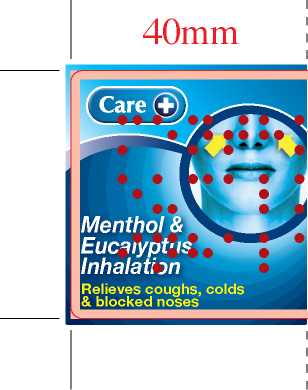
Menthol And Eucalyptus Inhalation Bp 1980
FRONT FACE
42mm
\
IMPORTANT: Peel this label at corner and read BEFORE use. Do not .ear off, re-fix foi future use. |
FOr ftmptomatic relief djcoughs, colds and blocked noses. Inhale the s.eamy vapour through the mouth and nose. SHAKE WELL bEFoRe USE. Adults, the elderly and children o.ji 3 months. Add 1 x 5ml •polhful tok568mk(#pim) of hot (not boiling) water. Inhale the vapour. Repeat after 4 hours if requited. Dtetttegive4k children under 3 monlhsoldunless your doltor tells you to. FOR EXTERNAL USE ONLY.
Peel where shown for further precautions.
Active ingredients:
menthol 2% w/v, eucalyptus oil 10% v/v.
Also contains: magnesium carbonate, benzalkonium chloride and purified water. Do not store above 25°C. Keep out of the sight and reach of children. Manufactured by the licence holder L.C.M. Ltd., Huddersfield, HD7 5QH, UK for Thornton and Ross Ltd., Huddersfield,
HD7 5QH, UK.
PL 12965/0027 21511009 £
100m l e
\
FRONT FACE REVERSE
110mm ! 40mm
i
|
\ | ||||
|
42mm |
This medicine contains menthol and eucalyptus oil which are decongestants and help ease breathing difficulties. 1. Before you use this medicine □ Do not use the medicine if you or your child have.... • An allergy to any of the ingredients listed on the front. • Or your child is under 3 months old. Pregnant or breastfeeding.. Using Menthol & Eucalyptus Inhalation during pregnancy and breastfeeding is unlikely to have any ill effects when used as directed. If unsure, talk to your doctor or pharmacist. 21511009 |
Important ingredient information • This product contains benzalkonium chloride which may cause difficulty in breathing or wheezing. 2. Possible side effects Like all medicines, Menthol & Eucalyptus Inhalation can have side effects, although these don’t affect everyone. Important side effects in infants: If you think your child has any of the following side effects or symptoms, stop using this medicine immediately and see a doctor as soon as possible. • Difficulty in breathing or collapse. | ||
|
V_ |
_/ | |||
CARRIER
40mm ! 110mm
i
|
\ | ||||
|
42mm |
Other possible side effects are: • Allergic reactions such as itching, swelling and blistering, including contact dermatitis. If you notice these or any other side effects, stop use and tell your doctor or pharmacist. They will tell you what to do. Reporting of side effects If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine. |
3. Further information If you accidentally swallow some see a doctor straight away. Take the pack with you to show which medicine you have swallowed. • Do not use after the expiry date. The expiry date refers to the last day of that month. • Return any unused medicine to the pharmacy for disposal. What the medicine looks like Menthol & Eucalyptus Inhalation is a cloudy white liquid which separates on standing. It is supplied in 100ml bottles. This label was last revised in February 2015 21511009 | ||
|
V |
/ | |||
Braille reads
|
• • |
• o |
• • |
o • |
• o |
• o |
• o |
o o |
o* |
• • |
oo |
oo |
oo |
oo |
o |
o |
|
o o |
o# |
o# |
• • |
• • |
o# |
• o |
o o |
oo |
• 0 |
oo |
oo |
oo |
oo |
o |
o |
|
• o m |
o o e |
• o n |
• o t |
° h |
• o o |
•l° |
o o |
oo •• & |
oo |
oo |
o o |
o o |
o |
o | |
|
• o |
• o |
• • |
• o |
• o |
• • |
• • |
o# |
• o |
o# |
oo |
oo |
oo |
oo |
o |
o |
|
o • |
oo |
O 0 |
oo |
• o |
o# |
• o |
• • |
O 0 |
• o |
oo |
oo |
0 o |
o o |
o |
o |
|
oo e |
• • u |
O 0 c |
oo a |
•l° |
• • y |
• o p |
• o t |
• • u |
• o s |
oo |
oo |
0 o |
0 o |
o |
o |
|
o* |
• • |
• 0 |
• o |
• o |
• o |
o# |
o* |
• 0 |
• • |
oo |
oo |
oo |
oo |
o |
o |
|
• o |
o • |
• • |
oo |
• o |
0 o |
• • |
• o |
o • |
o# |
oo |
oo |
0 o |
0 o |
o |
o |
|
0.0 1 |
• o n |
0,0 h |
oo a |
•l° |
0 o a |
•t° |
oo 1 |
• o o |
• o n |
oo |
oo |
0 o |
0 o |
o |
o |
MHRA Header Box
|
Product Title |
Menthol & Eucalyptus Inhalation |
Colours Used |
Fonts Used | |
|
□ |
Process Cyan |
Braille BQ | ||
|
Component |
Landscape peelable label |
□ |
Process Magenta |
Helvetica |
|
Pack Size |
100ml |
Process Yellow |
Helvetica Neue | |
|
IG Code |
21511009 |
■ |
Process Black |
OCRB |
|
Dimensions |
42 x 150mm |
□ |
Keyline (Non-printing) |
Optima |
|
Proof No. |
1 Date 11.02.2015 |
• |
Braille | |
|
Text free area (non-printing) | ||||