Methotrexate Astron 50 Mg/Ml Solution For Injection In Pre-Filled Syringe
PACKAGE LEAFLET: INFORMATION FOR THE USER
Methotrexate Astron 50mg/ml solution for injection in pre-filled syringe
methotrexate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Methotrexate is and what it is used for
2. What you need to know before you use Methotrexate
3. How to use Methotrexate
4. Possible side effects
5. How to store Methotrexate
6. Contents of the pack and other information
1. What Methotrexate is and what it is used for
Methotrexate contains methotrexate as active substance
Methotrexate is a substance with following properties:
• it interferes with the growth of certain cells in the body that reproduce quickly
• it reduces the activity of the immune system (the body’s own defence mechanism)
• it has anti-inflammatory effects
Methotrexate is indicated for the treatment of
• active rheumatoid arthritis in adult patients.
• polyarthritic forms of severe, active juvenile idiopathic arthritis, when the response to nonsteroidal anti-inflammatory drugs (NSAIDs) has been inadequate,
• severe recalcitrant disabling psoriasis, which is not adequately responsive to other forms of therapy such as phototherapy, PUVA, and retinoids, and severe psoriatic arthritis in adult patients.
• mild to moderate Crohn’s Disease in adult patients when adequate treatment with other medicines is not possible.
Rheumatoid arthritis (RA) is a chronic collagen disease, characterised by inflammation of the synovial membranes (joint membranes). These membranes produce a fluid which acts as a lubricant for many joints. The inflammation causes thickening of the membrane and swelling of the joint.
Juvenile arthritis concerns children and adolescents less than 16 years. Polyarthritic forms are indicated if 5 or more joints are affected within the first 6 months of the disease.
Psoriatic arthritis is a kind of arthritis with psoriatric lesions of the skin and nails, especially at the joints of fingers and toes.
Psoriasis is a common chronic skin disease, characterised by red patches covered by thick, dry, silvery, adherent scales.
Methotrexate modifies and slows down the progression of the disease.
Crohn’s Disease is a type of inflammatory bowel disease that may affect any part of the gastrointestinal tract causing symptoms such as abdominal pain, diarrhoea, vomiting or weight loss.
2. What you need to know before you use Methotrexate Do not use Methotrexate if you:
• are allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6),
• suffer from severe liver or kidney diseases or blood diseases.
• regularly drink large amounts of alcohol.
• suffer from a severe infection, e.g. tuberculosis, HIV or other immunodeficiency syndromes.
• suffer from ulcers in the mouth, stomach ulcer or intestinal ulcer.
• are pregnant or breast-feeding.
• receive vaccinations with live vaccines at the same time.
Warnings and precautions
Talk to your doctor or pharmacist or nurse before taking Methotrexate if:
• you are elderly or if you feel generally unwell and weak.
• you have problems with the way your liver works.
• you suffer from dehydration (water loss).
Recommended follow-up examinations and safety measures:
Even when Methotrexate is administered in low doses, severe side effects can occur. In order to detect them in time, check-ups and laboratory tests have to be carried out by your doctor.
Before therapy:
Before starting the treatment, blood samples will be taken in order to check that you have enough blood cells, tests to check your liver function, serum albumin (a protein in the blood) and kidney function. Your doctor will also check if you suffer from tuberculosis (infectious disease in combination with little nodules in the affected tissue) and a chest X-ray will be taken.
During therapy:
You will have the following tests at least once a month during the first six months and at least every three months thereafter:
• Examination of the mouth and throat for alterations of the mucosa
• Blood tests
• Check of liver function
• Check of kidney function
• Check of respiratory system and if necessary lung function test
Methotrexate may affect your immune system and vaccination results. It may also affect the result of immunological tests. Inactive, chronic infections (e.g. herpes zoster [shingles], tuberculosis, hepatitis B or C) may flare up. During therapy with Methotrexate you must not be vaccinated with live vaccines.
Radiation induced dermatitis and sun-burn can reappear under methotrexate therapy (recall-reaction). Psoriatic lesions can exacerbate during UV-irradiation and simultaneous administration of methotrexate.
Enlarged lymph nodes (lymphoma) may occur and therapy must then be stopped.
Diarrhoea can be a toxic effect of Methotrexate and requires an interruption of therapy. If you suffer from diarrhoea please speak to your doctor.
Encephalopathy (a brain disorder)/leukoencephalopathy (a special disorder of the white brain substance) have been reported in cancer patients receiving methotrexate therapy and cannot be excluded for methotrexate therapy in other disease.
Other medicines and Methotrexate
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The effect of the treatment may be affected if Methotrexate is administered at the same time as certain other drugs:
• Medicines harming the liver or the blood count, e.g. leflunomide
• Antibiotics (medicines to prevent/fight certain infections) such as: tetracyclines, chloramphenicol, and non-absorbable broad-spectrum antibiotics, penicillines, glycopeptides, sulphonamides (sulphur containing medicines that prevent/fight certain infections), ciprofloxacin and cefalotin
• Non-steroidal anti-inflammatory drugs or salicylates (medicines against pain and/or inflammation)
• Probenecid (medicine against gout)
• Weak organic acids like loop diuretics (“water tablets”) or some medicines used for treatment of pain and inflammatory diseases (e.g. acetylsalicylic acid, diclofenac and ibuprofen) and pyrazole (e.g. metamizol for treating pain)
• Medicinal products, which may have adverse effects on the bone marrow, e.g. trimethoprim-sulphamethoxazole (an antibiotic) and pyrimethamine
• Sulphasalazine (antirheumatic medicine)
• Azathioprine (an immunosuppressive agent sometimes used in severe forms of rheumatoid arthritis)
• Mercaptopurine (a cytostatic agent)
• Retinoids (medicine against psoriasis and other dermatological diseases)
• Theophylline (medicine against bronchial asthma and other lung diseases)
• Proton-pump inhibitors (medicines against stomach trouble)
• Hypoglycaemics (medicines that are used to lower the blood sugar)
Vitamins containing folic acid may impair the effect of your treatment and should only be taken when advised by your doctor.
Vaccination with live vaccine should be avoided.
Methotrexate with food, drink and alcohol
Alcohol as well as large amounts of coffee, caffeine-containing soft drinks and black tea should be avoided during treatment with Methotrexate.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You must not take Methotrexate during pregnancy. There is a risk of harm to the foetus and miscarriage. Men and women should use an effective method of birth control during treatment and during a further six months after treatment with Methotrexate has been discontinued.
In women of child-bearing age, any existing pregnancy must be excluded with certainty by taking appropriate measures, e.g. pregnancy test, prior to therapy.
As methotrexate can be genotoxic, all women who wish to become pregnant are advised to consult a genetic counselling centre, if possible, already prior to therapy, and men should seek advice about the possibility of sperm preservation before starting therapy.
Breast-feeding has to be stopped prior to and during treatment with Methotrexate.
Driving and using machines
Treatment with Methotrexate may cause adverse reactions affecting the central nervous system, e.g. tiredness and dizziness. Thus the ability to drive a vehicle and/or to operate machines may, in certain cases, be compromised. If you feel tired or drowsy you should not drive or use machines.
Methotrexate contains sodium
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially “sodium-free”.
3. How to use Methotrexate
Your doctor decides on the dosage, which is adjusted individually. Usually it takes 4 - 8 weeks before there is any effect of the treatment.
Methotrexate is administered subcutaneously (under the skin) by or under the supervision of a physician or healthcare staff as an injection once a week only. Together with your doctor you decide on a suitable weekday each week on which you receive your injection.
Use in children and adolescents
The doctor decides on the appropriate dose in children and adolescents with polyarthritic forms of juvenile idiopathic arthritis.
Methotrexate is not recommended in children less than 3 years of age due to insufficient experience in this age group.
Method and duration of administration Methotrexate is injected once weekly!
The duration of the treatment is determined by the treating physician. Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis vulgaris, psoriatic arthritis and Crohn’s disease with Methotrexate is a long-term treatment.
At the start of your therapy, Methotrexate may be injected by medical staff. In certain cases your doctor may decide to instruct you how to inject Methotrexate under the skin yourself. You will then receive appropriate training.
Under no circumstances should you try to inject Methotrexate yourself before you have received such training.
Please refer to the instructions for use at the end of the leaflet.
Please note that all of the contents have to be used.
The manner of handling and disposal must be consistent with that of other cytostatic preparations in accordance with local requirements. Pregnant health care personnel should not handle and/or administer Methotrexate.
Methotrexate should not come into contact with the surface of the skin or mucosa. In the event of contamination, the affected area must be rinsed immediately with plenty of water.
If you use more Methotrexate than you should
If you use more Methotrexate than you should, talk to your doctor immediately.
If you forget to use Methotrexate
Do not take a double dose to make up for a forgotten dose.
If you stop using Methotrexate
If you stop using Methotrexate, talk to your doctor immediately.
If you have the impression that the effect of Methotrexate is too strong or too weak, you should talk to your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency as well as the degree of severity of the side effects depends on the dosage level and the frequency of administration. As severe side effects may occur even at low dosage, it is important that you are monitored regularly by your doctor. Your doctor will do tests to check for abnormalities developing in the blood (such as low white blood cells, low platelets, lymphoma) and changes in the kidneys and the liver.
Tell your doctor immediately if you experience any of the following symptoms, as these may indicate a serious, potentially life-threatening side effect, which require urgent specific treatment:
• persistent dry, non-productive cough, shortness of breath and fever; these may be signs of an inflammation of the lungs (pneumonia) [common - may affect up to 1 in 10 people]
• symptoms of liver damage such as yellowing of the skin and whites of the eyes; methotrexate can cause chronic liver damage (liver cirrhosis), formation of scar tissue of the liver (liver fibrosis), fatty degeneration of the liver [all uncommon - may affect up to 1 in 100 people], inflammation of the liver (acute hepatitis) [rare - may affect up to 1 in
1.000 people] and liver failure [very rare - may affect up to 1 in 10,000 people]
• allergy symptoms such as skin rash including red itchy skin, swelling of the hands, feet, ankles, face, lips, mouth or throat (which may cause difficulty in swallowing or breathing) and feeling you are going to faint; these may be signs of severe allergic reactions or an anaphylactic shock [rare - may affect up to 1 in 1,000 people]
• symptoms of kidney damage such as swelling of the hands, ankles or feet or changes in frequency of urination or decrease or absence of urine; these may be signs of kidney failure [rare - may affect up to 1 in 1,000 people]
• symptoms of infections, e.g. fever, chills, achiness, sore throat; methotrexate can make you more susceptible to infections. Rarely [may affect up to 1 in 1,000 people] severe infections like a certain type of pneumonia (Pneumocystis carinii pneumonia) or blood poisoning (sepsis) may occur
• severe diarrhoea, vomiting blood and black or tarry stools; these symptoms may indicate a rare [may affect up to 1 in 1,000 people] severe complication of the gastrointestinal system caused by methotrexate e.g. gastrointestinal ulcers
• symptoms associated with the blockage (occlusion) of a blood vessel by a dislodged blood clot (thromboembolic event) such as weakness of one side of the body (stroke) or pain, swelling, redness and unusual warmth in one of your legs (deep vein thrombosis); methotrexate can cause thromboembolic events [rare - may affect up to 1 in
1.000 people]
• fever and serious deterioration of your general condition, or sudden fever accompanied by a sore throat or mouth, or urinary problems; methotrexate can very rarely [may affect up to 1 in 10,000 people] cause a sharp fall in white blood cells (agranulocytosis) and severe bone marrow suppression
• unexpected bleeding, e.g. bleeding gums, blood in the urine, vomiting blood or bruising, these can be signs of a severely reduced number of blood platelets caused by severe courses of bone marrow depression [very rare - may affect up to 1 in 10,000 people]
• severe skin rash or blistering of the skin (this can also affect your mouth, eyes and genitals); these may be signs of the very rare [may affect up to 1 in 10,000 people] conditions called Stevens Johnson syndrome or burned skin syndrome (toxic epidermal necrolysis)
In the following, please find the other side effects that may occur:
Very common: may affect more than 1 in 10 people
• Mouth inflammation, indigestion, nausea (feeling sick), loss of appetite
• Increase in liver enzymes
Common: may affect up to 1 in 10 people
• Mouth ulcers, diarrhoea
• Rash, reddening of the skin, itching
• Headache, tiredness, drowsiness
• Reduced blood cell formation with decrease in white and/or red blood cells and/or platelets (leukopenia, anaemia, thrombocytopenia)
Uncommon: may affect up to 1 in 100 people
• Throat inflammation, inflammation of the bowels, vomiting
• Increased sensitivity to light, loss of hair, increased number of rheumatic nodules, shingles, inflammation of blood vessels, herpes-like skin rash, hives
• Onset of diabetes mellitus
• Dizziness, confusion, depression
• Decrease in serum albumin
• Decrease in the number of blood cells and platelets
• Inflammation and ulcer of the urinary bladder or vagina, reduced kidney function, disturbed urination
• Joint pain, muscle pain, osteoporosis (reduction of bone mass)
Rare: may affect up to 1 in 1,000 people
• Increased skin pigmentation, acne, blue spots due to vessel bleeding
• Allergic inflammation of blood vessels, fever, red eyes, infection, wound-healing impairment, decreased number of anti-bodies in the blood
• Visual disturbances
• Inflammation of the sac around the heart, accumulation of fluid in the sac around the heart
• Low blood pressure
• Lung fibrosis, shortness of breath and bronchial asthma, accumulation of fluid in the sac around the lung
• Electrolyte disturbances
Very rare: may affect up to 1 in 10,000 people
• Profuse bleeding, toxic megacolon (acute toxic dilatation of the gut)
• Increased pigmentation of the nails, inflammation of the cuticles, furunculosis (deep infection of hair follicles), visible enlargement of small blood vessels
• • Impaired vision, pain, loss of strength or sensation of numbness or tingling in arms and legs, changes in taste (metallic taste), convulsions, paralysis, severe headache with fever
• Retinopathy (noninflammatory eye disorder)
• Loss of sexual drive, impotence, male breast enlargement (gynaecomastia), defective sperm formation, menstrual disorder, vaginal discharge
• Enlargement of lymphatic nodes (lymphoma)
Not Known: frequency cannot be estimated from the available data:
• Leukoencephalopathy (a disease of the white brain substance)
Subcutaneous application of methotrexate is locally well tolerated. Only mild local skin reactions (such as burning sensations, erythema, swelling, discolouration, severe itching, pain) were observed, decreasing during therapy.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Methotrexate
Keep this medicine out of the sight and reach of children.
Store below 30 °C.
Keep the pre-filled syringes in the outer carton in order to protect from light.
Do not use this medicine after the expiry date which is stated on the label/carton after EXP. The expiry date refers to the last day of that month.
Do not use Methotrexate if you notice sign of colour change or contain visible particles.
Do not throw away any medicines via waste water or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Methotrexate contains
• The active substance is methotrexate. 1 ml of solution contains methotrexate disodium corresponding to 50 mg methotrexate.
• The other ingredients are sodium chloride, sodium hydroxide (for pH adjustment) and water for injections.
What Methotrexate looks like and contents of the pack
Methotrexate pre-filled syringes contain a clear, yellow to brown solution.
The following pack sizes are available:
For 0.15 mL, 0.20 mL, 0.30 mL and 0.40 mL: packs of 1, 2, 4, 5, 6, 10, 12 and 24 prefilled syringes with fixed needle covered with rigid needle shield.
• For 0.25 mL, 0.35 mL, 0.45 mL, 0.55 mL and 0.60 mL: packs of 1, 4, 5, 6 and 12 prefilled syringes with fixed needle covered with rigid needle shield.
• For 0.50 mL: packs of 1, 2, 4, 5, 6, 10 and 12 prefilled syringes with fixed needle covered with rigid needle shield.
Pre-filled syringes are available with and without blister pack.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Astron Research Limited,
Sage House, 319 Pinner Road,
North Harrow,
Middlesex, HA1 4HF,
United Kingdom
Manufacturer:
Astron Research Limited,
Sage House, 319 Pinner Road,
North Harrow,
Middlesex, HA1 4HF,
United Kingdom
Wessling Hungary Kft.,
Foti ut 56., Budapest 1047,
Hungary
This leaflet was last revised in 01/2016.
Instructions for use
Carefully read the instructions below before starting your injection, and always use the injection technique advised by your doctor, pharmacist or nurse.
For any problem or question, contact your doctor, pharmacist or nurse.
Preparation
Select a clean, well-lit and flat working surface.
Collect necessary items before you begin:
• 1 Methotrexate pre-filled syringe
Wash your hands carefully. Before use, check the Methotrexate syringe for visual defects (or cracks).
Injection site
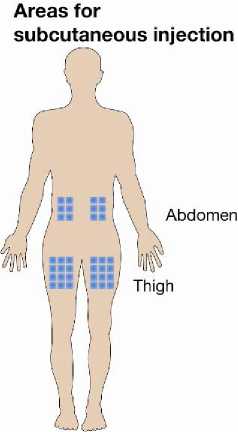
The best sites for injection are:
- upper thighs,
- abdomen except around the navel.
• If someone is helping you with the injection, he/she may also give the injection into the back of your arms, just below the shoulder.
• Change the injection site with each injection. This may reduce the risk of developing irritations at the injection site.
• Never inject into skin that is tender, bruised, red, hard, scarred or where you have stretch marks. If you have psoriasis, you should try not to inject directly into any raised, thick, red or scaly skin patches or lesions
Injecting the solution
1. Unpack the methotrexate pre-filled syringe and read the package leaflet carefully. Remove the pre-filled syringe from the packaging at room temperature.
2. Disinfection
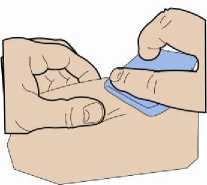
Choose an injection site and disinfect it with a swab soaked in disinfectant.
Allow at least 60 seconds for the disinfectant to dry.
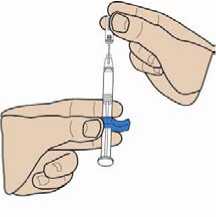
Carefully remove the protective cap by pulling it straight off the syringe. If the cap is very stiff, turn it slightly with a pulling movement.
3. Remove the protective cap
4. Inserting the cannula
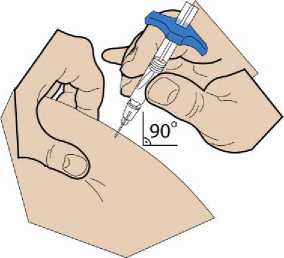
5. Injection
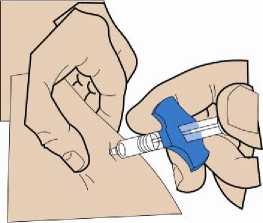
Important: Do not touch the needle of the pre-filled syringe!
Using two fingers, pinch up a fold of skin and quickly insert the needle into the skin at a 90-degree angle.
Insert the needle fully into the fold of skin. Push the plunger down slowly and inject all of the liquid underneath your skin. Hold the skin securely until the injection is completed. Pre-filled syringe is not intended to deliver partial doses.
Carefully pull the needle straight out.
Methotrexate should not come into contact with the surface of the skin or mucosa. In the event of contamination, the affected area must be rinsed immediately with plenty of water.
If you or someone around you is injured by the needle, consult your doctor immediately and do not use this pre-filled syringe.
Disposal and other handling
The manner of handling and throwing away of the medicine and pre-filled syringe must be in consistent with that of other cytostatic preparations in accordance with local requirements. Pregnant healthcare personnel should not handle and/or administer Methotrexate.