Metoclopramide Hydrochloride 5Mg/5Ml Oral Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Metoclopramide Hydrochloride 5mg/5ml Oral Solution
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 5ml of oral solution contains 5mg metoclopramide hydrochloride.
Excipient(s) with known effect:
Methyl parahydroxybenzoate - 5mg/5ml Propyl parahydroxybenzoate - 1mg/5ml Sorbitol solution (non-crystallising) - 0.25ml/5ml
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Oral Solution
4.1. Therapeutic Indications
Adult population
Metoclopramide is indicated in adults for:
- Prevention of delayed chemotherapy induced nausea and vomiting (CINV).
- Prevention of radiotherapy induced nausea and vomiting (RINV).
- Symptomatic treatment of nausea and vomiting, including acute migraine induced nausea and vomiting. Metoclopramide can be used in combination with oral analgesics to improve the absorption of analgesics in acute migraine.
Paediatric population
Metoclopramide is indicated in children (aged 1-18 years) for:
- Prevention of delayed chemotherapy induced nausea and vomiting (CINV) as a second line option.
4.2. Posology and Method of Administration
Method of Administration.
For oral use.
Suitable for administration via nasogastric (NG) or percutaneous endoscopic gastrostomy (PEG) tubes. For further instructions see section 6.6.
Posology.
All indications (adult patients)
The recommended single dose is 10 mg, repeated up to three times daily.
The maximum recommended daily dose is 30 mg or 0.5 mg/kg body weight. The maximum recommended treatment duration is 5 days.
Prevention of delayed chemotherapy induced nausea and vomiting (CINV) (paediatric patients aged 1-18 years)
The recommended dose is 0.1 to 0.15 mg/kg body weight, repeated up to three times daily by oral route. The maximum dose in 24 hours is 0.5 mg/kg body weight.
Dosing table
|
Age |
Body Weight |
Dose |
Frequency |
|
1-3 years |
10-14 kg |
1 mg (1ml) |
Up to 3 times daily |
|
3-5 years |
15-19 kg |
2 mg (2ml) |
Up to 3 times daily |
|
5-9 years |
20-29 kg |
2.5 mg (2.5ml) |
Up to 3 times daily |
|
9-18 years |
30-60 kg |
5 mg (5ml) |
Up to 3 times daily |
|
15-18 years |
Over 60 kg |
10 mg (10ml) |
Up to 3 times daily |
The maximum treatment duration is 5 days for prevention of delayed chemotherapy induced nausea and vomiting (CINV).
Method of administration:
A minimal interval of 6 hours between two administrations is to be respected, even in case of vomiting or rejection of the dose (see section 4.4).
Special population
Elderly
In elderly patients a dose reduction should be considered, based on renal and hepatic function and overall frailty.
Renal impairment:
In patients with end stage renal disease (Creatinine clearance < 15 ml/min), the daily dose should be reduced by 75%.
In patients with moderate to severe renal impairment (Creatinine clearance 1560 ml/min), the dose should be reduced by 50% (see section 5.2).
Hepatic impairment:
In patients with severe hepatic impairment, the dose should be reduced by 50% (see section 5.2).
Paediatric population
Metoclopramide is contraindicated in children aged less than 1 year (see section 4.3).
4.3. Contraindications
- Hypersensitivity to metoclopramide hydrochloride or any of the excipients listed in section 6.1
- Patients hypersensitive to procaine and procainamide may show crosssensitivity
- Metoclopramide should not be used during the first three to four days following operations such as pyloroplasty or gut anastomosis as vigorous muscular contractions may not help healing
- Gastrointestinal haemorrhage, mechanical obstruction or gastrointestinal perforation for which the stimulation of gastrointestinal motility constitutes a risk
- Confirmed or suspected pheochromocytoma, due to the risk of severe hypertension episodes
- History of neuroleptic or metoclopramide-induced tardive dyskinesia
- Epilepsy (increased crises frequency and intensity)
- Parkinson’s disease
- Combination with levodopa or dopaminergic agonists (see section 4.5)
- Known history of methaemoglobinaemia with metoclopramide, or of NADH cytochrome-b5 deficiency
- Use in children less than 1 year of age due to an increased risk of extrapyramidal disorders (see section 4.4).
4.4. Special warnings and precautions for use
If, despite treatment, vomiting persists, the patient must be re-assessed to exclude the possibility of an underlying disorder, i.e. cerebral irritation.
Care should be exercised when using metoclopramide in patients with a history of atopy (including asthma) or porphyria.
Patients receiving this drug for the disorders associated with delayed gastric emptying should be reviewed at an early stage for response to treatment.
Metoclopramide may cause elevation of serum prolactin levels.
Neurological Disorders
Extrapyramidal disorders may occur, particularly in children and young adults, and/or when high doses are used. These reactions occur usually at the beginning of the treatment and can occur after a single administration. Metoclopramide should be discontinued immediately in the event of extrapyramidal symptoms. These effects are generally completely reversible after treatment discontinuation, but may require a symptomatic treatment (benzodiazepines in children and/or anticholinergic anti-Parkinsonian medicinal products in adults).
The time interval of at least 6 hours specified in section 4.2 should be respected between each metoclopramide administration, even in case of vomiting and rejection of the dose, in order to avoid overdose.
Prolonged treatment with metoclopramide may cause tardive dyskinesia, potentially irreversible, especially in the elderly. Treatment should not exceed 3 months because of the risk of tardive dyskinesia (see section 4.8). Treatment must be discontinued if clinical signs of tardive dyskinesia appear.
Neuroleptic malignant syndrome has been reported with metoclopramide in combination with neuroleptics as well as with metoclopramide monotherapy (see section 4.8). Metoclopramide should be discontinued immediately in the event of symptoms of neuroleptic malignant syndrome and appropriate treatment should be initiated.
Special care should be exercised in patients with underlying neurological conditions and in patients being treated with other centrally-acting drugs (see section 4.3).
Symptoms of Parkinson’s disease may also be exacerbated by metoclopramide.
Methaemoglobinemia
Methaemoglobinemia which could be related to NADH cytochrome b5 reductase deficiency has been reported. In such cases, metoclopramide should be immediately and permanently discontinued and appropriate measures initiated (such as treatment with methylene blue).
Cardiac Disorders
There have been reports of serious cardiovascular undesirable effects including cases of circulatory collapse, severe bradycardia, cardiac arrest and QT prolongation following administration of metoclopramide by injection, particularly via the intravenous route (see section 4.8).
Special care should be taken when administering metoclopramide, particularly via the intravenous route to the elderly population, to patients with cardiac conduction disturbances (including QT prolongation), patients with uncorrected electrolyte imbalance, bradycardia and those taking other drugs known to prolong QT interval.
Intravenous doses should be administered as a slow bolus (at least over 3 minutes) in order to reduce the risk of adverse effects (e.g. hypotension, akathisia).
Renal and Hepatic Impairment
In patients with renal impairment or with severe hepatic impairment, a dose reduction is recommended (see section 4.2).
Excipient Warnings
• This product contains sorbitol. Patients with rare hereditary problems of fructose intolerance should not take this medicine.
• Methyl and propyl parahydroxybenzoates are contained in this product which may cause allergic reactions (possibly delayed).
4.5. Interactions with other medicinal products and other forms of interaction
Contraindicated combination
Levodopa or dopaminergic agonists (including apomorphine, bromocriptine and pergolide) and metoclopramide have a mutual antagonism (see section 4.3).
Combination to be avoided
Alcohol potentiates the sedative effect of metoclopramide; concurrent use may also accelerate gastric emptying of alcohol and thus may promote the rate and extent of absorption from the small intestine.
Combination to be taken into account
Due to the prokinetic effect of metoclopramide, the absorption of certain drugs may be modified.
Anticholinergics and morphine derivatives
Anticholinergics and morphine derivatives may have both a mutual antagonism with metoclopramide on the digestive tract motility.
Central nervous system depressants (morphine derivatives, anxiolytics, sedative H1 antihistamines, sedative antidepressants, barbiturates, clonidine and related)
Sedative effects of Central Nervous System depressants and metoclopramide are potentiated.
Neuroleptics
Metoclopramide may have an additive effect with other neuroleptics on the occurrence of extrapyramidal disorders.
Serotonergic drugs
The use of metoclopramide with serotonergic drugs such as SSRIs may increase the risk of serotonin syndrome.
Digoxin
Metoclopramide may decrease digoxin bioavailability. Careful monitoring of digoxin plasma concentration is required.
Ciclosporin
Metoclopramide increases ciclosporin bioavailability (Cmax by 46% and exposure by 22%). Careful monitoring of ciclosporin plasma concentration is required. The clinical consequence is uncertain.
Mivacurium and suxamethonium
Metoclopramide injection may prolong the duration of neuromuscular block (through inhibition of plasma cholinesterase).
Strong CYP2D6 inhibitors
Metoclopramide exposure levels are increased when co-administered with strong CYP2D6 inhibitors such as fluoxetine and paroxetine. Although the clinical significance is uncertain, patients should be monitored for adverse reactions.
Extrapyramidal reaction causing drugs (such as phenothiazines and tetrabenazine) Concurrent use with metoclopramide may increase the frequency and severity of extrapyramidal side effects. Care should be exercised in the event of co-administration of these drugs.
Mexiletine
Concurrent use with metoclopramide may accelerate absorption of mexiletine. Diagnostic interference
With Gonadorelin test, concurrent use with metoclopramide may blunt the response to gonaderelin by increasing serum prolactin concentrations. Concurrent metoclopramide therapy may increase aldosterone and serum prolactin levels.
Aspirin and paracetamol
The absorption of any concurrently administered oral drug may be modified by the effect of metoclopramide on gastric motility. Drugs known to be affected in this way include aspirin and paracetamol.
Atovaquone
Metoclopramide may reduce plasma concentrations of atovaquone.
4.6. Fertility, pregnancy and lactation
Pregnancy
A large amount of data on pregnant women (more than 1000 exposed outcomes) indicates neither malformative toxicity nor foetotoxicity. Metoclopramide can be used during pregnancy if clinically needed. Due to pharmacological properties (as with other neuroleptics), in case of metoclopramide administration at the end of pregnancy, extrapyramidal syndrome in newborn cannot be excluded. Metoclopramide should be avoided at the end of pregnancy. If metoclopramide is used, neonatal monitoring should be undertaken.
Breastfeeding
Metoclopramide is excreted in breast milk at low level. Adverse reactions in the breast-fed baby cannot be excluded. Therefore metoclopramide is not recommended during breastfeeding. Discontinuation of metoclopramide in breastfeeding women should be considered.
4.7. Effects on Ability to Drive and Use Machines
Metoclopramide may cause drowsiness, dizziness, dyskinesia and dystonias which can affect the vision and also interfere with the ability to drive and operate machinery.
4.8. Undesirable Effects
Adverse reactions listed by System Organ Class. Frequencies are defined using the following convention: very common (>1/10), common (>1/100, <1/10), uncommon (>1/1000, <1/100), rare (>1/10000, <1/1000), very rare (<1/10000), not known (cannot be estimated from the available data).
|
System Organ Class |
Frequency |
Adverse reactions |
|
Blood and lymphatic system disorders | ||
|
Not known |
Methaemoglobinaemia, which could be related to NADH cytochrome b5 | |
|
reductase deficiency, particularly in neonates (see section 4.4); Sulfhaemoglobinaemia, mainly with concomitant administration of high doses of sulfur-releasing medicinal products | ||
|
Cardiac disorders | ||
|
Uncommon |
Bradycardia, particularly with intravenous formulation | |
|
Not known |
Cardiac arrest, occurring shortly after injectable use, and which can be subsequent to bradycardia (see section 4.4); Asystole; Atrioventricular block; Sinus arrest particularly with intravenous formulation; Electrocardiogram QT prolonged; Torsade de Pointes | |
|
Endocrine disorders* | ||
|
Uncommon |
Amenorrhoea; Hyperprolactinaemia | |
|
Rare |
Galactorrhoea | |
|
Not known |
Gynaecomastia | |
|
Eye disorders | ||
|
Not known |
Visual disturbances have been reported | |
|
Gastrointestinal disorders | ||
|
Common |
Diarrhoea | |
|
Rare |
Constipation; Nausea; Unusual dryness of mouth | |
|
General disorders and administration site conditions | ||
|
Common |
Asthenia | |
|
Rare |
Oedema (including face oedema) | |
|
Immune system disort |
ers | |
|
Uncommon |
Hypersensitivity | |
|
Not known |
Anaphylactic reaction (including anaphylactic shock particularly with intravenous formulation) | |
|
Nervous system disorc |
ers | |
|
Very common |
Somnolence | |
|
Common |
Extrapyramidal disorders (particularly in children and young adults and/or when the recommended dose is exceeded, even following administration of a single dose of the drug) (see section 4.4); Parkinsonism; Akathisia | |
|
Uncommon |
Dystonia; Dyskinesia; Depressed level of consciousness | |
|
Rare |
Convulsion especially in epileptic patients; Dizziness ; Headache | |
|
Not known |
Tardive dyskinesia which may be persistent, during or after prolonged treatment, particularly in elderly patients | |
|
(see section 4.4); Neuroleptic malignant syndrome (see section 4.4) | ||
|
Psychiatric disorders | ||
|
Common |
Depression; Restlessness | |
|
Uncommon |
Hallucination | |
|
Rare |
Confusional state; Trouble sleeping; Unusual irritability | |
|
Respiratory, thoracic and mediastinal disorders | ||
|
Not known |
Dyspnoea may occur | |
|
Skin and subcutaneous tissue disorders | ||
|
Rare |
Skin rash; A small number of skin reactions such as urticaria and pruritus | |
|
Vascular disorder | ||
|
Common: |
Hypotension; particularly with intravenous formulation | |
|
Not known |
Shock, Syncope after injectable use, Acute hypertension in patients with phaeochromocytoma (see section 4.3) | |
* Endocrine disorders during prolonged treatment in relation with hyperprolactinaemia (amenorrhoea, galactorrhoea, gynaecomastia).
The following reactions, sometimes associated, occur more frequently when high doses are used:
- Extrapyramidal symptoms: acute dystonia and dyskinesia, parkinsonian syndrome, akathisia, even following administration of a single dose of the medicinal product, particularly in children and young adults (see section 4.4).
- Drowsiness, decreased level of consciousness, confusion, hallucination.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
4.9. Overdose
Symptoms
Extrapyramidal Disorder (muscle spasms, especially of jaw, neck, back, shuffling walk, tic like (jerky) movements of head and face, trembling and shaking of hands), drowsiness, decreased level of consciousness, confusion, hallucination and cardio-respiratory arrest may occur.
Management
In case of extrapyramidal symptoms related or not to overdose, the treatment is only symptomatic (benzodiazepines in children and/or anticholinergic antiparkinsonian medicinal products in adults).
A symptomatic treatment and a continuous monitoring of the cardiovascular and respiratory functions should be carried out according to clinical status.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Metoclopramide is a dopaminergic blocker, acting as a delayed gastrointestinal emptying adjunct and as a peristaltic stimulant. The exact mechanism of action is unknown; it is believed that metoclopramide inhibits gastric smooth muscle relaxation produced by dopamine thus enhancing cholinergic responses of the gastrointestinal smooth muscles. Accelerates intestinal transit and gastric emptying by preventing relaxation of gastric body and increasing the phasic activity of antrum. At the same time, this action is accompanied by relaxation of the upper small intestine, resulting in an improved co-ordination between the body and the antrum of the stomach and the upper small intestine. Decreases reflux into the oesophagus by increasing the resting pressure of the lower oesophageal sphincter and improves acid clearance from the oesophagus by increasing amplitude of oesophageal peristaltic contractions.
As an anti-emetic; the dopamine antagonist action raises the threshold of activity in the chemoreceptor trigger zone and decreases the input from afferent visceral nerves.
Metoclopramide also stimulates prolactin secretion.
5.2. Pharmacokinetic properties
Animal studies have shown metoclopramide to bind to plasma protein (13% -22%), especially plasma albumin. Biotransformation is by the hepatic route.
Metoclopramide has a half life of four to six hours. The onset of action, by oral route of administration, is from 30 to 60 minutes. The duration on action is 1 to 2 hours.
Elimination is by the renal route, approximately 85% of an oral dose appears in the urine as unchanged drug and as sulfate and glucuronide conjugates.
Renal impairment
The clearance of metoclopramide is reduced by up to 70% in patients with severe renal impairment, while the plasma elimination half-life is increased (approximately 10 hours for a creatinine clearance of 10-50 mL/minute and 15 hours for a creatinine clearance <10 mL/minute).
Hepatic impairment
In patients with cirrhosis of the liver, accumulation of metoclopramide has been observed, associated with a 50% reduction in plasma clearance.
5.3. Pre-clinical Safety Data
None stated.
6.1. List of excipients
Methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), propylene glycol (E1520), sorbitol solution (non crystallising) (E420), glycerol (E422), citric acid monohydrate (E330), lime and lemon flavours, sodium citrate (E331) and purified water.
6.2. Incompatibilities
Not applicable.
6.3. Shelf life
24 months
1 month once opened
6.4. Special Precautions for Storage
Store below 25°C and protect from light.
6.5. Nature and contents of container
Bottle: Amber Type III glass bottle
Capacity: 150ml
Closures: HDPE, EPE wadded, tamper evident, child resistant
Syringe: Polypropylene body and purple HDPE plunger with a capacity
of 10ml, graduated at each 1ml and intermediate marks every
0.5ml
Bottle adaptor: Low density polyethylene
6.6. Special precautions for disposal and other handling
Keep out of the sight and reach of children.
Any unused product or waste material should be disposed of in accordance with local requirements.
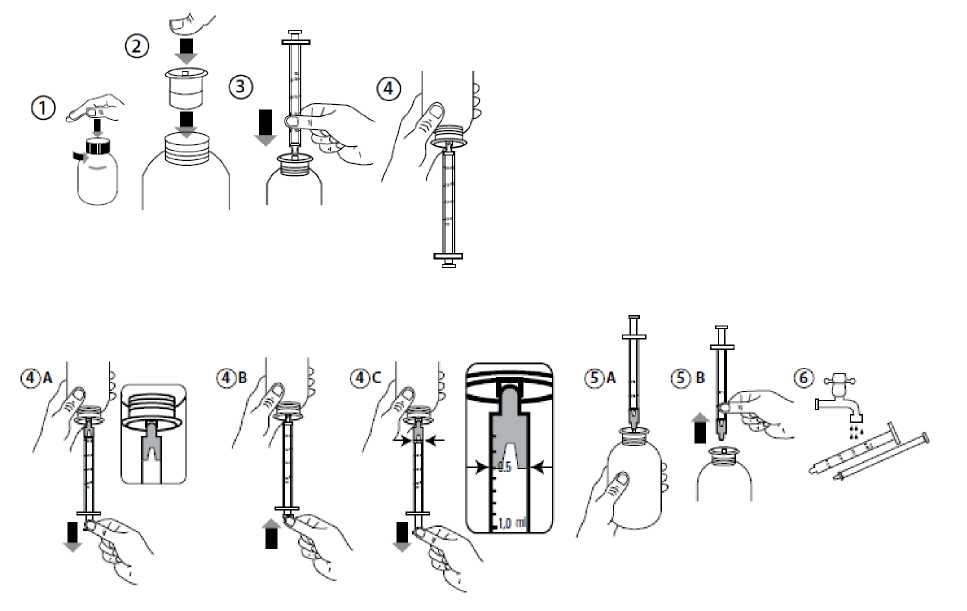
Instructions for using the oral dosing syringe:
• Open the bottle: press the cap and turn it anticlockwise (Figure 1).
• Insert the syringe adaptor into the bottle neck (Figure 2).
• Take the syringe and put it in the adaptor opening (Figure 3).
• Turn the bottle upside down (Figure 4).
• Fill the syringe with a small amount of solution by pulling the piston down (Figure 4A). Then push the piston upward in order to remove any possible bubbles (Figure 4B). Finally, pull the piston down to the
graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (Figure 4C).
• Turn the bottle the right way up (Figure 5A).
• Remove the syringe from the adaptor (Figure 5B).
• Put the end of the syringe into your mouth and push the piston slowly back in to take the medicine.
• Wash the syringe with water and let it dry before you use it again (Figure 6).
• Close the bottle with the plastic screw cap - leave the syringe adaptor in the bottle.
Instruction for administration via nasogastric (NG) or percutaneous endoscopic gastrostomy (PEG) tubes:
Ensure that the enteral feeding tube is free from obstruction before administration.
1. Flush the enteral tube with 5mL of water.
2. Administer the required dose of Metoclopramide Hydrochloride Oral Solution with a suitable measuring device.
3. Flush the enteral tube with 5mL of water.
This product has not been tested with latex NG or PEG tubes and therefore should not be used with tubes made from latex.
7 MARKETING AUTHORISATION HOLDER
Rosemont Pharmaceuticals Ltd
Rosemont House
Yorkdale Industrial Park
Braithwaite Street
Leeds
LS11 9XE
UK
8. MARKETING AUTHORISATION NUMBER
PL 00427/0117
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
Date of first authorisation: 1 July 1998 Date of Renewal: 15 June 2006
10 DATE OF REVISION OF THE TEXT
02/01/2016