Mhl Benzocaine Sore Throat Spray 1.5 Mg Oromucosal Spray
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
MHL Benzocaine Sore Throat Spray 1.5 mg Oromucosal Spray
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Benzocaine 1.5 mg per actuation.
For full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Oromucosal spray, solution (Oromucosal spray)
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Symptomatic temporary relief of pain associated with sore throat pain and minor mouth infections.
4.2 Posology and method of administration
Adults (including elderly):
Spray two metered doses every two to three hours if required (not more than sixteen doses in every 24 hours) or as directed by the physician.
Children aged 6 and over:
One metered dose every two to three hours if required (not more than eight doses in every 24 hours) or as directed by the physician.
Not suitable for children under 6 years.
Route of administration
Topical application to the mucosa of the mouth and throat by means of a metered dose aerosol.
Product should not be administered for more than 7 consecutive days. The container should be shaken before use.
Fig. 1
Resting position (cannot be activated)
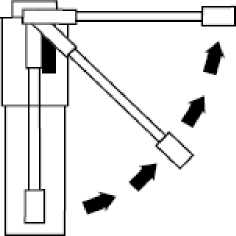
Fig. 3
Hold your head upright, insert the nozzle well into your mouth and press the plunger once to deliver a single metered dose,
Fig. 2
Raise nozzle from vertical to horizontal position
Before use it may be necessary to press the plunger 2-3 times to activate spray.
4.3 Contraindications
Known hypersensitivity to benzocaine. Methaemoglobinaemia
Special warnings and precautions for use
4.4
This preparation should not be administered to children under 6 years or used for more than seven consecutive days unless directed by a physician.
If the sore throat is severe, persistent or accompanied by fever or headache, a physician should be consulted before the use of this product. Avoid spraying into eyes.
Caution should be exercised in the use of this product if there have been previous allergic reactions with other local anaesthetics or sunscreen products.
Avoid inhalation of the product.
To be used with caution in patients with asthma.
4.5 Interaction with other medicinal products and other forms of interaction
Benzocaine is an ester which on hydrolysis produces p-aminobenzoic acid so it should not be used in patients being treated with sulphonamides.
4.6 Pregnancy and lactation
There is no evidence, at present, of hazard from benzocaine in pregnancy. However, only very limited data is available. Therefore it should not be used in pregnancy unless considered essential by a physician.
4.7 Effects on ability to drive and use machines
None.
4.8
Undesirable effects
Hypersensitivity reactions to benzocaine have been reported.
Methaemoglobinaemia may occur in patients receiving high doses or repeated applications of benzocaine-containing products.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
4.9 Overdose
There have been no reports of overdosage with MHL Benzocaine Sore Throat Spray. Systemic effects are unlikely.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
ATC Code: R02AD01
Benzocaine is a surface anaesthetic of the ester type. It has found frequent use as lozenges or solution to treat pain arising from various throat and mouth conditions.
5.2 Pharmacokinetic properties
Benzocaine is sparingly soluble in water with toxicity about a tenth that of cocaine. It is not readily absorbed from mucus membranes. It is an ester which on hydrolysis produces p-aminobenzoic acid.
5.3 Preclinical safety data
None stated.
6.1 List of excipients
Cetylpyridinium chloride
glycerine
ethanol
clove bud oil
menthol crystals
sodium saccharin 450
peppermint
Cremophor RH40
water
6.2 Incompatibilities
None.
6.3 Shelf life
24 months.
6.4 Special precautions for storage
Do not store above 25 °C
6.5 Nature and contents of container
White aluminium can with 100 mcl pumps, white high density polypropylene
folding arm activator and white high density polyethylene cap.
Pack size: not less than 7.3 g (approximately 60 metered doses).
6.6 Special precautions for disposal
To help protect the environment, do not dispose of this medicine via wastewater or household waste. Ask a pharmacist for advice on disposal.
7 MARKETING AUTHORISATION HOLDER
Manx Pharma Limited Taylor Group House Wedgnock Lane Warwick CV34 5YA United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
15833/0059
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10/10/2012
10 DATE OF REVISION OF THE TEXT
09/06/2015