Mirena Intra-Uterine System
Mirena® Intra-Uterine System Patient Information Leaflet
(levonorgestrel)
This product is known by the above name, but will be referred to as Mirena throughout the remainder of the leaflet
About this leaflet
Please read this leaflet carefully before you decide to have Mirena fitted.
It provides you with some useful information about Mirena. The information in this leaflet applies only to Mirena. If you have any questions or are not sure about anything, please ask your doctor or nurse.
In this leaflet:
1. What Mirena is and what it is used for
2. Before you have Mirena fitted
3. How and when Mirena is used
4. Possible side effects
5. Howto store Mirena
6. Further information
1. What Mirena is and what it is used for
Mirena is an intrauterine system (IUS) placed inside the womb (uterus) where it slowly releases the hormone levonorgestrel. It can be used in the following three ways:
1. As an effective long-term and reversible method of contraception.
2. For reducing menstrual blood flow, if you suffer from heavy periods (heavy menstrual bleeding).
It can be used for contraception and heavy menstrual bleeding until it is removed or up to a maximum of 5 years.
3. If you are going through the menopause Mirena can be used in conjunction with an oestrogen as part of a hormone replacement therapy (HRT) regimen to protect the lining of your womb.
Not so much is known about how well Mirena protects the lining of the womb beyond 4 years of use in women who are taking oestrogen to treat menopausal symptoms. Therefore, if you are using it in this way, your doctor or nurse will remove your Mirena after 4 years. Your doctor will be able to advise you further.
Children and adolescents
Mirena is not indicated for use before the first menstrual bleeding (menarche).
How does Mirena work?
As a contraceptive:
The hormone in Mirena prevents pregnancy by:
• controlling the monthly development of the womb lining so that it is not thick enough for you to become pregnant
• making the mucus in the opening to the womb (the cervical canal) thicker, so that the sperm cannot get through to fertilise the egg
• preventing the release of eggs (ovulation) in some women.
There are also some effects on the lining of the womb caused by the presence of the T-shaped frame of the Mirena device.
In the treatment of heavy menstrual bleeding:
The hormone in Mirena reduces menstrual bleeding by controlling the monthly development of the womb lining, making it thinner, so that there is less bleeding every month.
As part of an HRT regimen:
The menopause is a gradual process which usually takes place between the ages of about 45 and 55. Although the menopause is natural, it often causes distressing symptoms such as hot flushes and night sweats. These symptoms are due to the gradual loss of the female sex hormones (oestrogen and progestogen) produced by the ovaries.
Oestrogens can be used to relieve the menopausal symptoms. However, taking oestrogens alone increases the risk of abnormal growth or cancer of the lining of the womb. Taking a progestogen, such as the hormone in Mirena (levonorgestrel), as part of an HRT regimen lowers this risk by protecting the lining of the womb.
2. Before you have Mirena fitted
Your doctor or nurse will carry out some tests before you have Mirena fitted to make sure that it is suitable for you to use. This will include a pelvic examination so that pregnancy and sexually transmitted diseases can be excluded and may also include other examinations such as a breast examination, if your doctor or nurse feels this is appropriate.
Genital infections will need to be successfully treated before you can have Mirena fitted.
If Mirena is to be fitted for HRT use your doctor will firstly carry out an assessment of your symptoms to ensure that treatment is only initiated for symptoms that adversely affect your quality of life. Such an assessment should be repeated by your doctor at least annually. You should also consult the Patient Information Leaflet of the oestrogen product that is to be used in conjunction with Mirena before starting your HRT regimen as there are some important risk factors associated with HRT that you should consider, such as the risk of endometrial cancer, breast cancer and blood clots. You may feel pain or have some bleeding during insertion.
If you have epilepsy, tell the doctor or nurse fitting the Mirena because, although rare, a fit can occur during insertion. Some women might feel faint after the procedure. This is normal and your doctor or nurse will tell you to rest for a while.
Do not use Mirena and please tell your doctor or nurse if you:
• are pregnant or suspect that you may be pregnant
• have or have had any type of cancer or suspected cancer including blood cancer (leukaemia) unless in remission, uterine, cervical and breast cancer
• currently have or have had recurrent pelvic inflammatory disease
• have or have had inflammation of the neck of the womb (cervix)
• have an unusual or unpleasant vaginal discharge, or vaginal itching as this may indicate an infection
• have or have had inflammation of the lining of your womb following delivery of your baby
• have or have had an infection of the womb after delivery or after abortion during the past 3 months
• have any condition which makes you susceptible to infections. A doctor will have told you if you have this
• have or have had an abnormal smear test (changes in the cervix)
• have undiagnosed vaginal bleeding
• have an abnormal womb or abnormal growths in the womb (fibroids) which distort the uterine cavity
• have or have had liver problems
• have or have had trophoblastic disease. A doctor will have told you if you have this
• are sensitive to the hormone levonorgestrel or to any of the ingredients in Mirena (see section 5 ‘What Mirena contains’).
Mirena must not be used as part of an HRT regimen if you have had a stroke, heart attack or any heart problems.
Mirena may not be suitable for all women.
Consult your doctor or nurse if you:
• have or develop migraine with visual disturbances, unusually bad headaches or if you have headaches more often than before
• have yellowing of the skin or whites of the eyes (jaundice)
• have high blood pressure
• have had a cancer affecting your blood (including leukaemia) which is now in remission
• are on long-term steroid therapy
• have ever had a previous ectopic pregnancy (pregnancy outside the womb)
• have a history of fluid filled sacks in the ovary (ovarian cysts)
• are having Mirena fitted for contraception or heavy menstrual bleeding and have had a stroke or heart attack, or if you have any heart problems
• disease of your arteries (arterial disease)
• have a history of blood clots (thrombosis)
• are diabetic, as Mirena may affect glucose tolerance.
You may still be able to use Mirena if you have or have had some of these conditions.
Your doctor or nurse will advise you.
You must also tell your doctor or nurse if any of these conditions occur for the first time while you have Mirena in place.
You must see a doctor or nurse as soon as possible if you develop painful swelling in your leg, sudden chest pain or difficulty breathing as these may be a sign of a blood clot. It is important that any blood clots are treated promptly.
You must also see a doctor without delay if you develop persistent lower abdominal pain, fever, pain during sexual intercourse or abnormal bleeding. If you get severe pain or fever shortly after Mirena has been inserted, you may have a severe infection which must be treated immediately.
It is advisable to give up smoking when using hormone containing products such as Mirena.
Can I change my mind?
Your doctor or nurse can remove Mirena at any time. Unless you wish to get pregnant the removal should be carried out during the first few days of your period. Otherwise it is important to use another form of contraception (e.g. condoms) in the 7 days leading up to the removal as intercourse during this week could lead to pregnancy after Mirena is removed.
If you do wish Mirena to be removed so that you can get pregnant your usual level of fertility is expected to return after it is removed. Studies have suggested that in women who discontinue Mirena (in order to become pregnant) the pregnancy rate at one year is similar to those who do not use contraception.
Taking other medicines
The effect of hormonal contraceptives such as Mirena may be reduced by medicines that increase the amounts of enzymes made by the liver. Please tell your doctor or nurse if you are taking:
• phenobarbital, primidone, phenytoin or carbamazepine (to treat epilepsy)
• griseofulvin (an antifungal)
• rifampicin or rifabutin (antibiotics)
• nevirapine or efavirenz (for HIV).
Please tell your doctor or nurse if you are taking or have recently taken any other medicines, including medicines obtained without prescription.
Pregnancy and breastfeeding
Mirena should not be used during pregnancy or if you think you are pregnant.
It is very rare for women to become pregnant with Mirena in place.
Missing a period may not mean that you are pregnant as some women may not have periods at all while using Mirena. However, in order to exclude the possibility of pregnancy, you should consider a pregnancy test if you have not had a period for 6 weeks. If this test is negative there is no need to carry out another test, unless you have other signs of pregnancy, e.g. sickness, tiredness or breast tenderness.
If you do become pregnant with Mirena in place, please contact your doctor as soon as possible so that ectopic pregnancy can be excluded and Mirena removed to reduce the risk of spontaneous abortion.
Very small amounts of the hormone in Mirena are found in breast milk but the levels are lower than with any other hormonal
contraceptive method. Please ask your doctor or nurse for advice before breastfeeding.
3. How and when Mirena is used
Only a doctor or specially trained nurse can fit Mirena. They will explain the fitting procedure and any risks associated with its usage. You will then be examined by your doctor or nurse before Mirena is fitted. If you have any concerns over its usage you should discuss it with them.
When Mirena is fitted for contraception or heavy menstrual bleeding:
Mirena should be inserted either during your period or within seven days from the beginning of your period. If you already have Mirena and it is time to replace it with a new one, you do not need to wait until your period. If you have just had a baby, you should wait at least 6 weeks before having Mirena fitted (see section 4 ‘Possible side effects - Severe pain and continued bleeding’). Mirena can sometimes be fitted immediately after you have had an abortion, provided that you have no genital infections.
When Mirena is fitted for HRT use:
If you no longer have periods then Mirena can be inserted at any time. If you still have periods, Mirena should be inserted during the last days of bleeding.
Remind your healthcare provider that you have Mirena inserted, especially if they were not the person who inserted it.
How quickly does Mirena work?
Contraception:
You are protected from pregnancy as soon as Mirena is fitted. The possibility of becoming pregnant is approximately 2 in 1,000 in the first year. The failure rate may increase in case of the Mirena coming out by itself or perforation (see section 4 ‘Possible side effects’).
Heavy menstrual bleeding:
Mirena usually results in lighter periods after 3 to 6 months of treatment.
HRT use:
The hormone in Mirena will begin to protect the lining of your womb as soon as it is fitted.
How often should I have Mirena checked?
You should have it checked 6 weeks after it is fitted. Your doctor may determine how often and what kind of check-ups are required in your particular case.
How can I tell whether Mirena is in place?
Gently put a finger into your vagina and feel for the two thin threads attached to the lower end of Mirena. Your doctor or nurse will show you howto do this.
Do not pull the threads because you may accidentally pull it out. If you cannot feel the threads, contact your doctor or nurse as soon as possible and in the meantime avoid intercourse or use a barrier contraceptive (such as condoms). The threads may have simply drawn up into the womb or cervical canal. If the threads still cannot be found by your doctor or nurse, they may have broken off, or Mirena may have come out by itself, or in rare cases it may have perforated the wall of your womb (uterine perforation, see section 4). It may be necessary for you to have an ultrasound scan or x-ray to locate Mirena.
Contact your doctor or nurse if you can feel the lower end of Mirena itself or you or your partner feel pain or discomfort during sexual intercourse.
PP2/1425/Patient/V1
What happens if Mirena comes out by itself?
If it comes out either completely or partially you may not be protected against pregnancy.
It is rare but possible for this to happen without you noticing during your menstrual period. An unusual increase in the amount of bleeding during your period might be a sign that this has happened. Tell your doctor or nurse if there are any unexpected changes in your bleeding pattern.
How will Mirena affect my periods?
Mirena will affect your menstrual cycle.
For all uses of Mirena:
You may have lighter periods or painful periods or some spotting (light bleeding in between periods) and irregular bleeding during the first few months after Mirena is fitted.
You may have prolonged or heavy bleeding or an increase in the frequency of bleeding, usually in the first 2 to 3 months, before a reduction in blood loss is achieved.
Overall you are likely to have fewer days bleeding in each month and you might eventually have no periods at all. This is due to the effect of the hormone (levonorgestrel) on the lining of the womb.
If you have had Mirena fitted for heavy menstrual bleeding:
You should have lighter periods after 3 to 6 months. If you do not have lighter periods after 3 to 6 months, alternative treatments should be considered.
If you have had Mirena fitted for HRT use:
If you have had Mirena fitted for quite a long time and then start to have bleeding problems, it is important that you contact your
doctor so that tests can be carried out to exclude changes to your womb.
There is a calendar on the last page of this patient information leaflet. Your doctor or nurse may ask you to fill this in to check your pattern of bleeding. If you are asked to do so, mark the date of insertion with an “X” in the appropriate date square. Mark days of spotting with “o” and bleeding with
4. Possible side effects
Taking any medicine carries some risk of side effects. With Mirena these are most common during the first months after it is fitted and decrease as time goes on.
If you experience any of the following serious side effects please contact your doctor or nurse immediately:
• Severe pain or fever developing shortly after insertion may
mean that you have a severe infection which must be treated immediately. In rare cases very severe infection (sepsis) can occur.
• Severe pain and continued bleeding as this might be a sign of damage or tear in the wall of the womb (perforation). Perforation is rare, but occurs most often during the fitting of the Mirena, although the perforation may not be detected until sometime later. If this happens the Mirena will be removed; very rarely this may require surgery. The risk of perforation is low, but increased in breastfeeding women and in women who have had a baby up to 36 weeks before insertion.
Possible signs and symptoms of perforation may include:
• severe pain (like menstrual cramps) or more pain than expected
• heavy bleeding (after insertion)
• pain or bleeding which continues for more than a few weeks
• sudden changes in your periods
• pain during sex
• you can no longer feel the Mirena threads (see section 3 ‘How and when Mirena is used - How can I tell whether Mirena is in place?’)
• Lower abdominal pain especially if you also have a fever or have missed a period or have unexpected bleeding, as
this might be a sign of ectopic pregnancy. The absolute risk of ectopic pregnancy in Mirena users is low. However, when a woman becomes pregnant with Mirena in place, the relative likelihood of ectopic pregnancy is increased.
• Lower abdominal pain or experience painful or difficult sex as this might be a sign of ovarian cysts or pelvic inflammatory disease. This is important as pelvic infections can reduce your chances of having a baby and can increase the risk of ectopic pregnancy.
Very Common (more than 1 in 10 women)
• vaginal bleeding including spotting
• absent, light or infrequent menstrual periods
Common (less than 1 in 10 women)
• ovarian cysts
• painful periods
• weight gain
• depression, nervousness
• headache
• migraine
• abdominal, pelvic or back pain
• nausea
• acne
• increased growth of hair on the face and body
• reduced sex drive
• increased vaginal discharge
• inflammation of the vulva and vagina
• tender, painful breasts
• Mirena coming out by itself
Uncommon (less than 1 in 100 women)
• genital infections that may cause: vaginal itching; pain on passing urine; or lower abdominal pain from inflammation of the womb, ovaries or Fallopian tubes
• infection or inflammation of the lining of the womb, which may cause a foul smelling vaginal discharge (endometritis)
• inflammation of the neck of the womb (cervicitis)
• swelling of your abdomen, legs or ankles
• hair loss
• itchy skin including eczema
• skin discolouration/increased skin pigment especially on the face (chloasma)
Rare (less than 1 in 1000 women)
• rashes
• uterine perforation (see ‘serious side effects’ above)
Unknown frequency
• allergic reaction (symptoms may include rash, itching or rapid swelling of the face, mouth, tongue and/or throat)
• increased blood pressure
Your partner may feel the removal threads during intercourse.
Every woman is at risk of breast cancer, but it is rare in women under the age of 40. Breast cancer has been reported in Mirena users, although the risk and frequency are unknown.
In pre-menopausal women, the frequency of developing breast cancer whilst using Mirena is possibly similar to that associated with using Combined Oral Contraceptives, but the evidence for this is less conclusive.
In post-menopausal women, using hormone replacement therapy (HRT) slightly increases the risk of breast cancer. Although the risk of developing breast cancer is higher with combined oestrogen/progestogen HRT, than with oestrogen-only HRT, the risk of breast cancer developing when Mirena is prescribed to provide the progestogen component of HRT is not yet known. The patient information leaflet of the oestrogen component of the treatment should also be consulted for additional information.
It is important to regularly check your breasts and you should contact your doctor if you feel any lump in your breasts. You should also tell your doctor if a close relative has or ever had breast cancer.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or nurse.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.aov.uk/vellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Mirena
Keep out of the sight and reach of children.
Do not use intra-uterine device after expiry date stated on the box. No special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer required. This will help to protect the environment.
6. Further Information
Mirena contains 52 milligrams of levonorgestrel. The hormone is contained within a substance called polydimethylsiloxane.
This is surrounded by a membrane (skin) also made of polydimethylsiloxane. The Mirena T-shaped frame also contains barium sulphate so that it can be seen on X-rays.
What Mirena looks like and contents of the pack
Mirena consists of a small T-shaped frame made from a plastic called polyethylene. There are two fine threads, made of iron oxide and polyethylene, attached to the bottom of the frame. These allow easy removal and allow you or your doctor or nurse to check that Mirena is in place.
Each sterile pack contains one Mirena and should not be opened until required.
Manufactured by: BAYER OY, PANSIONTIE 47, 20210 TURKU, FINLAND. Procured from within the EU. Product Licence Holder: Quadrant Pharmaceuticals Ltd, Lynstock House, Lynstock Way, Lostock, Bolton BL6 4SA. Repackaged by Maxearn Ltd, Bolton BL6 4SA.
Mirena Intra-Uterine System PL 20774/1425 Mirena is a registered trademark of Bayer Oy
Date of preparation 30th August 2016
PP2/1425/Patient/V1
POM
Date of insertion = X Spotting = O Bleeding = •
|
Month 1 | ||||||
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
\E |
M |
0 | ||||
|
Month 2 | |
|
0 0 0 0 0 0 |
0 |
|
0 0 0 0 0 0 |
0 |
|
0 0 0 0 0 0 |
21 |
|
0 0 0 0 0 0 |
28 |
|
0 0 0 |
Patient Information
Patient Name:.........
Date of Fitting:........
Doctor’s Name:.......
Telephone No:........
First check-up visit:..
Next visit:
1.
2.
3.
4.
5.
Mirena Intra-Uterine System
(levonorgestrel)
Medical Information Leaflet
Therapeutic indications
Contraception. Idiopathic menorrhagia. Mirena may be particularly useful in women with idiopathic menorrhagia requiring (reversible) contraception. Protection from endometrial hyperplasia during oestrogen replacement therapy.
Posology and method of administration Starting treatment
• Contraception and idiopathic menorrhagia
In women of fertile age, Mirena is inserted into the uterine cavity within seven days of the onset of menstruation. It can be replaced by a new system at any time of the cycle.
Post-partum insertion: To reduce the risk of perforation, postpartum insertions should be postponed until the uterus is fully involuted. Do not insert earlier than six weeks after delivery. If the patient is experiencing significant post-partum bleeding and/or pain then infection or other causes should be excluded before insertion. Mirena can also be inserted immediately after the first trimester abortion.
Mirena is effective for 5 years in the indications for contraception and idiopathic menorrhagia so should be removed after 5 years use. If the user wishes to continue using the same method, a new system can be inserted at the same time, in which case no additional protection is required.
If pregnancy is not desired, the removal should be carried out during the first few days after the onset of the woman’s menstruation. Otherwise contraception has to be ensured with other methods (e.g. condoms) starting at least 7 days before the removal.
• Protection from endometrial hyperplasia during oestrogen replacement therapy.
When used for endometrial protection during oestrogen replacement therapy, Mirena can be inserted at any time in an amenorrhoeic woman, or during the last days of menstruation or withdrawal bleeding.
In the indication for protection from endometrial hyperplasia during oestrogen replacement therapy, clinical data (from clinical trials conducted in women of 18 years and over) beyond 4 years of use are limited. Mirena should therefore be removed after 4 years.
Mirena provides the progestogen component of hormone therapy (HRT). Therefore in women receiving HRT, Mirena can be used in combination with oral or transdermal oestrogen preparations without additional exogenous progestogens. The product information of the oestrogen component of the HRT should be consulted prior to the use of Mirena as the important risk factors associated with HRT use should be considered, such as the risk of endometrial cancer, breast cancer and venous thromboembolisms.
Instructions for use and handling
Mirena is supplied in a sterile pack which should not be opened until required for insertion. The exposed product should be handled with aseptic precautions. If the seal of the sterile package is broken, the product should be discarded (see ‘Special precautions for disposal’).
• How to Insert Mirena
It is strongly recommended that Mirena should only be inserted by physicians/healthcare professionals who are experienced in Mirena insertions and/or have undergone sufficient training for Mirena insertion.
In case of difficult insertion and/or exceptional pain or bleeding during or after insertion, please refer to ‘Special warnings and precautions for use: Insertion/removal warnings and precautions.’ Please see the ‘Insertion instructions’ section on a separate leaflet.
• How to Remove Mirena
Please see the ‘Insertion instructions’ section on a separate leaflet.
• Paediatric population
There are no relevant indications for use of Mirena before menarche.
• Geriatric patients
Mirena has not been studied in women over the age of 65 years.
• Patients with hepatic impairment
Mirena is contraindicated in women with acute liver disease or liver tumour (see ’Contraindications').
• Patients with renal impairment
Mirena has not been studied in women with renal impairment.
PP1/1425/Medical/V6
Contraindications
Known or suspected pregnancy; confirmed or suspected hormone dependent tumours including breast cancer; current or recurrent pelvic inflammatory disease; cervicitis; current genital infection; postpartum endometritis, infected abortion during the past three months; conditions associated with increased susceptibility to infections; cervical dysplasia; uterine or cervical malignancy; undiagnosed abnormal genital bleeding; congenital or acquired abnormality of the uterus including fibroids if they distort the uterine cavity; liver tumour or other acute or severe liver disease; acute malignancies affecting the blood or leukaemias except when in remission; recent trophoblastic disease while hCG levels remain elevated; hypersensitivity to the active substance or to any of the excipients.
Active or previous severe arterial disease, such as stroke or myocardial infarction is a contraindication when Mirena is used in conjunction with an oestrogen for HRT use.
Special warning and precautions for use Medical Examination
Before insertion, a complete personal and family medical history should be taken. Physical examination should be guided by this and by the contraindications and warnings for use. Pulse and blood pressure should be measured and a bimanual pelvic examination performed to establish the orientation of the uterus. The patient should be re-examined six weeks after insertion and further examinations should be performed where clinically indicated and adapted to the individual woman rather than as routine procedure. Prior to insertion pregnancy should be excluded and genital infection should be successfully treated. Women should be advised that Mirena does not protect against HIV (AIDs) and other sexually transmitted disease (please refer to the section below on pelvic infections).
Women should be encouraged to attend cervical and breast screening as appropriate for their age.
For the treatment of postmenopausal symptoms, HRT should only be initiated for symptoms that adversely affect quality of life. In all cases, a careful appraisal of the risks and benefits should be undertaken at least annually and HRT should only be continued as long as the benefit outweighs the risk. The contraindications and warnings for the oestrogen component should also be considered prior to commencing the HRT regimen.
Conditions under which Mirena can be used with caution
Should any of the following conditions exist or arise for the first time during treatment, removal of the system should be considered:
• Migraine with aura
• Unusually severe or unusually frequent headache
• Jaundice
• Marked increase of blood pressure
• Malignancies affecting the blood or leukaemias in remission
• Use of chronic corticosteroid therapy
• Past history of symptomatic functional ovarian cysts
• Active or previous severe arterial disease, such as stroke or myocardial infarction (see ‘Contraindications’ when Mirena is used in conjunction with an oestrogen for HRT use).
• Severe or multiple risk factors for arterial disease
• Thrombotic arterial or any current embolic disease
• Acute venous thromboembolism.
In general, women using hormonal contraception should be encouraged to give up smoking.
Mirena should be used with caution in postmenopausal women with advanced uterine atrophy.
Insertion/removal warnings and precautions
• General Information:
As the insertion technique is different from other intrauterine devices, special emphasis should be given to training in the correct insertion technique. Instructions for insertion are in the package.
Insertion and removal may be associated with some pain and bleeding. In case of difficult insertion and/or exceptional pain or bleeding during or after insertion, physical examination and ultrasound should be performed immediately to exclude perforation of the uterine corpus or cervix (see also Perforation). Physical examination alone (including checking of threads) may not be sufficient to exclude partial perforation.
The procedure may precipitate fainting as a vasovagal reaction, or a seizure in an epileptic patient. In the event of early signs of a vasovagal attack, insertion may need to be abandoned or the system removed. The woman should be kept supine, the head lowered and the legs elevated to the vertical position if necessary in order to restore cerebral blood flow. A clear airway must be maintained; an airway should always be at hand. Persistent bradycardia may be controlled with intravenous atropine. If oxygen is available it may be administered.
Table 1: Incidence of perforation per 1000 insertions for the entire study cohort, stratified by breastfeeding and time since delivery at insertion (parous women)
|
Breastfeeding at time of insertion |
Not breastfeeding at time of insertion | |
|
Insertion < 36 weeks after delivery |
5.6 (95% Cl 3.9-7.9; n=6047 insertions) |
1.7 (95% Cl 0.8-3.1; n=5927 insertions) |
|
Insertion > 36 weeks after delivery |
1.6 (95% Cl 0.0-9.1; n=608 insertions) |
0.7 (95% Cl 0.5-1.1; n=41,910 insertions) |
• Perforation:
Perforation of the uterine corpus or cervix may occur, most commonly during insertion, although it may not be detected until sometime later. This may be associated with severe pain and continued bleeding. If perforation is suspected the system should be removed as soon as possible; surgery may be required.
In a large prospective comparative non-interventional cohort study in IUD users (N = 61,448 women), the incidence of perforation was 1.3 (95% Cl:
1.1 - 1.6) per 1000 insertions in the entire study cohort; 1.4 (95% Cl 1.1 — 1.8 per 1000 insertions in the Mirena cohort and 1.1 (95% Cl: 0.7 -1.6) per 1000 insertions in the copper IUD cohort.
The study showed that both breastfeeding at the time of insertion and insertion up to 36 weeks after giving birth were associated with an increased risk of perforation (see Table 1). These risk factors were independent of the type of IUD inserted.
The risk of perforation may be increased in women with a fixed retroverted uterus.
Re-examination after insertion should follow the guidance given above under the heading "Medical examination" above, which may be adapted as clinically indicated in women with risk factors for perforation.
• Pelvic infection:
The insertion tube helps to prevent Mirena from contamination with microorganisms during the insertion and the Mirena inserter has been designed to minimise the risk of infections. In users of copper intrauterine devices (lUDs), the highest rate of pelvic infections occurs during the first month after insertion and decreases later. Known risk factors for pelvic inflammatory disease are multiple sexual partners, frequent intercourse and young age. Pelvic infection may have serious consequences as it may impair fertility and increase the risk of ectopic pregnancy. As with other gynaecological or surgical procedures, severe infection or sepsis (including group A streptococcal sepsis) can occur following I US insertion, although this is extremely rare. For women using Mirena with symptoms and signs suggestive of pelvic infection, bacteriological examinations are indicated and monitoring is recommended, even with discrete symptoms, and appropriate antibiotics should be started. There is no need to remove Mirena unless the symptoms fail to resolve within the following 72 hours or unless the woman wishes Mirena to be removed. Mirena must be removed if the woman experiences recurrent endometritis or pelvic infection, or if an acute infection is severe.
Complications leading to failure
• Expulsion:
Symptoms of the partial or complete expulsion of any IUS may include bleeding or pain. However, a system can be expelled from the uterine cavity without the woman noticing it. Partial expulsion may decrease the effectiveness of Mirena. As the system decreases menstrual flow, increase of menstrual flow may be indicative of an expulsion. A displaced Mirena should be removed and a new system inserted.
The woman should be advised how to check the threads of Mirena.
• Lost threads:
If the retrieval threads are not visible at the cervix on follow-up examination - first exclude pregnancy. The threads may have been drawn up into the uterus or cervical canal and may reappear during the next menstrual period. If they cannot be found, they may have broken off, the system may have been expelled, or rarely the device may be extrauterine after having perforated the uterus. An ultrasound should be arranged to locate the device and alternative contraception should be advised in the mean time. If an ultrasound cannot locate the device and there is no evidence of expulsion, a plain abdominal X-ray should be performed to exclude an extrauterine device.
Bleeding irregularities
• Irregular bleeding:
Mirena usually achieves a significant reduction in menstrual blood loss in 3 to 6 months of treatment. Increased menstrual flow or unexpected bleeding may be indicative of expulsion. If menorrhagia persists then the woman should be re-examined. An assessment of the uterine cavity should be performed using ultrasound scan. An endometrial biopsy should also be considered.
Risk in pre-menopausal women
Because irregular bleeding/spotting may occur during the first months of therapy in pre-menopausal women, it is recommended to exclude endometrial pathology before insertion of Mirena.
Risk in post-menopausal women
If the woman continues the use of Mirena inserted earlier for contraception, endometrial pathology has to be excluded if bleeding disturbances appear after commencing oestrogen replacement therapy. If bleeding irregularities develop during a prolonged treatment, appropriate diagnostic measures should also be taken as irregular bleeding may mask symptoms and signs of endometrial polyps or cancer.
• When to check for pregnancy in women of child bearing potential:
The possibility of pregnancy should be considered if menstruation does not occur within six weeks of the onset of previous menstruation and expulsion should be excluded. A repeated pregnancy test is not necessary in amenorrhoeic subjects unless indicated by other symptoms. In a study in women who used Mirena for contraception (n=130), oligomenorrhoea and amenorrhoea were reported in 57% and 16% of women respectively at the end of the first year of use.
• Treatment review advice for Menorrhagia:
Mirena usually achieves a significant reduction in menstrual blood loss in 3 to 6 months of treatment. If significant reduction in blood loss is not achieved in these time-frames, alternative treatments should be considered.
Other risks during use
• Ectopic pregnancy
The absolute risk of ectopic pregnancy in Mirena users is low. However, when a woman becomes pregnant with Mirena in situ, the relative likelihood of ectopic pregnancy is increased. The possibility of ectopic pregnancy should be considered in the case of lower abdominal pain - especially in connection with missed periods or if an amenorrhoeic woman starts bleeding. In a large prospective comparative non-interventional cohort study with an observation period of 1 year, the ectopic pregnancy rate with Mirena was 0.02%. In clinical trials, the absolute rate of ectopic pregnancy in users of Mirena was approximately 0.1% per year. This rate is lower than the rate of 0.3-0.5 % per year estimated for women not using any contraception. Women with a previous history of ectopic pregnancy carry a higher risk of a further ectopic pregnancy.
• Ovarian Cysts
Since the contraceptive effect of Mirena is mainly due to its local effect, ovulatory cycles with follicular rupture usually occur in women of fertile age. Sometimes atresia of the follicle is delayed and folliculogenesis may continue. These enlarged follicles cannot be distinguished clinically from ovarian cysts.
Data from clinical trials suggest that ovarian cysts have been reported as an adverse drug reaction in approximately 7% of women using Mirena, however some published studies have reported a higher incidence of ovarian cysts (which could have been influenced by factors including frequency and criteria of ultrasound scanning, and patient population). Most of these follicles are asymptomatic, although some may be accompanied by pelvic pain or dyspareunia. In most cases, the ovarian cysts disappear spontaneously during two to three months’ observation. Should this not happen, continued ultrasound monitoring and other diagnostic/therapeutic measures are recommended. Rarely, surgical intervention may be required.
• Breast cancer
Risk in pre-menopausal women
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using combined oral contraceptives (COCs), mainly using oestrogen-progestogen preparations. The excess risk gradually disappears during the course of the 10 years after cessation of COC use. Because breast cancer is rare in women under 40 years of age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer.
The risk of having breast cancer diagnosed in users of progestogen-only methods (POPs, implants and injectables), including Mirena, is possibly of similar magnitude to that associated with COC. However, for progestogen-only contraceptive preparations, the evidence is based on much smaller populations of users and so is less conclusive than that for COCs.
Risk in post-menopausal women
The risk of breast cancer is increased in post-menopausal women using systemic (i.e. oral or transdermal) hormone replacement therapy (HRT).
This risk is higher with combined oestrogen-progestogen HRT than with oestrogen-only HRT. The risk of breast cancer when Mirena is prescribed to provide the progestogen component of HRT is not yet known. The product information of the oestrogen component of the treatment should also be consulted for additional information.
General Information
• Glucose tolerance
Low-dose levonorgestrel may affect glucose tolerance, and the blood glucose concentration should be monitored in diabetic users of Mirena.
• Post-coital contraception
Limited experience suggests that Mirena is not suitable for use as a post-coital contraceptive.
Interaction with other medicinal products and other forms of interaction
The metabolism of progestogens may be increased by concomitant use of substances known to induce drug-metabolising enzymes, specifically cytochrome P450 enzymes, such as anticonvulsants (e.g. phenobarbital, primidone, phenytoin, carbamazepine) and anti-infectives (e.g., griseofulvin, rifampicin, rifabutin, nevirapine, efavirenz). The influence of these drugs on the contraceptive efficacy of Mirena has not been studied but is not believed to be of major importance due to the local mechanism of action.
Fertility, pregnancy and lactation
• Pregnancy
The use of Mirena during an existing or suspected pregnancy is contraindicated. In case of an accidental pregnancy with Mirena in situ, ectopic pregnancy should be excluded (see ‘Special warnings and precautions for use’) and the system must be removed and termination of the pregnancy should be considered. Removal of Mirena or probing of the uterus may result in spontaneous abortion. Should these procedures not be possible, the woman should be informed about increased risk of spontaneous abortion or premature labour observed during the use of copper and plastic lUDs. Accordingly, such pregnancies should be closely monitored. The woman should be instructed to report all symptoms that suggest complications of the pregnancy, like cramping abdominal pain with fever.
Because of the intrauterine administration and the local exposure to the hormone, teratogenicity (especially virilisation) cannot be completely excluded. It can be expected that the systemic hormone exposure of the foetus through the maternal circulation is lower than with any other hormonal contraceptive method. Clinical experience of the outcomes of pregnancies with Mirena in situ is limited. However, the woman should be informed that, to date, there is no evidence of birth defects caused by Mirena use in cases where pregnancy continues to term with Mirena in place.
• Lactation
Levonorgestrel has been identified in the breast milk. About 0.1% of the levonorgestrel dose is transferred during breast-feeding, but it is not likely that there will be a risk for the child with the dose released from Mirena, when it is inserted in the uterine cavity.
There appears to be no deleterious effects on infant growth or development when using any progestogen-only method after six weeks postpartum. Progestogen-only methods do not appear to affect the quantity or quality of breast milk. Uterine bleeding has rarely been reported in women using Mirena during lactation.
• Fertility
Studies have suggested that in women who discontinue Mirena for planned pregnancy the pregnancy rate at one year is similar to those who do not use contraception.
Undesirable effects
Undesirable effects are more common during the first months after the insertion, and subside during prolonged use.
Very common undesirable effects (occurring in more than 10% of users) include uterine/vaginal bleeding including spotting, oligomenorrhoea, amenorrhoea (see ‘Pharmacodynamic properties’).
The frequency of benign ovarian cysts depends on the diagnostic method used (see ‘Special warnings and precautions for use’) but has been estimated from clinical trial data to occur in 7% of users.
Cases of sepsis (including group A streptococcal sepsis) have been reported following IUD insertion (see ‘Special warnings and precautions for use’).
When a woman becomes pregnant with Mirena in situ, the relative risk of ectopic pregnancy is increased.
Cases of breast cancer have been reported in Mirena users.
The following adverse reactions have been reported in connection with the insertion or removal procedure of Mirena: pain, bleeding and insertion-related vasovagal reaction with dizziness or syncope. The procedure may also precipitate a seizure in patients with epilepsy. The removal threads may be felt by the partner during intercourse.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at:www.mhra. gov.uk/yellowcard.
(See table opposite)
Overdose
Not applicable
Pharmacodynamic properties
ATC Code: G02BA03
Pharmacotherapeutic group: Plastic IUD with progestogen Levonorgestrel is a progestogen used in gynaecology in various ways: as the progestogen component in oral contraceptives, in hormonal replacement therapy or alone for contraception in minipills and subdermal implants.
Levonorgestrel can also be administered directly into the uterine cavity as an intrauterine system. This allows a very low daily dosage, as the hormone is released directly into the target organ.
The contraceptive mechanism of action of Mirena is based on mainly hormonal effects producing the following changes:
• Prevention of proliferation of the endometrium
• Thickening of the cervical mucus thus inhibiting the passage of sperm
• Suppression of ovulation in some women.
The physical presence of the system in the uterus would also be expected to make a minor contribution to its contraceptive effect.
When inserted according to the insertion instructions, Mirena has a contraceptive failure rate of approximately 0.2% (95% Cl: 0.1-0.3) per year and a cumulative failure rate of approximately 0.7% at 5 years. The failure rate may increase in case of Mirena expulsion or perforation.
Mirena may be particularly useful for contraception in patients with excessive menstrual bleeding, and can be successfully used in the treatment of idiopathic menorrhagia. Results from three comparative studies indicate that in menorrhagic women, menstrual blood loss decreased by 62-94% at the end of three months and by 71-95% at the end of six months of use. Mirena appears to have similar effects to endometrial ablation/resection in reducing the menstrual blood loss up to two years. Menorrhagia caused by submucosal fibroids may respond less favourably. Reduced bleeding promotes the increase of blood haemoglobin in patients with menorrhagia.
In idiopathic menorrhagia, prevention of proliferation of the endometrium is the probable mechanism of action of Mirena in reducing blood loss.
The efficacy of Mirena in preventing endometrial hyperplasia during continuous oestrogen treatment is the same when oestrogen is administered orally ortransdermally. The observed hyperplasia rate under oestrogen therapy alone is as high as 20%. In clinical studies with a total of 634 perimenopausal and postmenopausal users of Mirena, no cases of endometrial hyperplasia were reported up to four years.
Bleeding Patterns:
Different kinds of bleeding changes (frequent, prolonged or heavy bleeding, spotting, oligomenorrhoea, amenorrhoea) are experienced by all users of Mirena. In fertile women the average number of spotting days/month decreases gradually from nine to four days during the first six months of use. The percentage of women with prolonged bleeding (more than eight days) decreases from 20% to 3% during the first three months of use. In clinical studies during the first year of use, 17% of women experienced amenorrhoea of at least three months duration.
When used in combination with oestrogen replacement therapy, perimenopausal users of Mirena may experience spotting and irregular bleeding during the first months of the treatment. The amount of bleeding becomes minimal during the first year, and 30-60% of users are totally free of bleedings.
Special precautions for disposal
Mirena is supplied in a sterile pack which should not be opened until required for insertion. Each system should be handled with aseptic precautions. If the seal of the sterile envelope is broken, the system inside should be disposed of in accordance with the local guidelines for the handling of biohazardous waste. Likewise, a removed Mirena and inserter should be disposed of in this manner. The outer carton package and the inner blister package can be handled as household waste.
Procured from within the EU. Product Licence Holder: Quadrant Pharmaceuticals Ltd, Lynstock House, Lynstock Way, Lostock, Bolton,
BL6 4SA. Repackaged by Maxearn Ltd, Bolton, BL6 4SA.
PL 20774/1425
Date of preparation 30th August 2016
PP2/1425/Medical/V1
|
System Organ Class |
Common >1/100 to <1/10 |
Uncommon >1/1000 to <1/100 |
Rare >1/10,000 to <1/1000 |
Unknown |
|
Immune system disorders |
Hypersensitivity including rash, urticaria and angioedema | |||
|
Psychiatric disorders |
Depressed mood/ Depression Nervousness Decreased libido | |||
|
Nervous system disorders |
Headache Migraine | |||
|
Gastrointestinal disorders |
Abdominal pain Nausea |
Abdominal distension | ||
|
Skin and subcutaneous tissue disorders |
Acne Hirsutism |
Alopecia Pruritus Eczema Chloasma/Skin Hyperpiqmentation |
Rash | |
|
Musculoskeletal, Connective tissue and bone disorders |
Back pain | |||
|
Reproductive system and breast disorders |
Ovarian cysts Pelvic pain Dysmenorrhoea Vaginal discharge Vulvovaginitis Breast tenderness Breast pain |
Pelvic inflammatory disease Endometritis Cervicitis / Papanicolaou smear normal, class II |
Uterine perforation1 | |
|
General disorders and administration site conditions |
Intrauterine contraceptive device expelled |
Oedema | ||
|
Investigations |
Weight increase |
Blood pressure increased |
Mirena® Intra-Uterine System
Insertion Instructions
This product is known by the above name, but will be referred to as Mirena throughout the remainder of the leaflet
Only to be inserted by a trained healthcare professional using aseptic technique.
Mirena is supplied within an inserter in a sterile package which should not be opened until needed for insertion.
Do not resterilise. As supplied, Mirena is for single use only. Do not use if the inner package is damaged or open. Do not insert after the expiry month and year shown on the label.
For timing of insertion, please consult the Mirena prescribing information.
Preparation for insertion
• Examine the patient to establish the size and position of the uterus, in order to detect any signs of acute genital infections or other contraindications for the insertion of Mirena and to exclude pregnancy.
• Insert a speculum, visualise the cervix, and then thoroughly cleanse the cervix and vagina with a suitable antiseptic solution.
• Use an assistant as necessary.
• Grasp the anterior lip of the cervix with a tenaculum or other forceps to stabilise the uterus. If the uterus is retroverted, it may be more appropriate to grasp the posterior lip of the cervix. Gentle traction on the forceps can be applied to straighten the cervical canal. The forceps should remain in position and gentle counter traction on the cervix should be maintained throughout the insertion procedure.
• Advance a uterine sound through the cervical canal to the fundus to measure the depth and confirm the direction of the uterine cavity and to exclude any evidence of intrauterine abnormalities (e.g. septum, submucous fibroids) or a previously inserted intrauterine contraceptive which has not been removed. If difficulty is encountered, consider dilatation of the canal. If cervical dilatation is required, consider using analgesics and/or a paracervical block.
Insertion
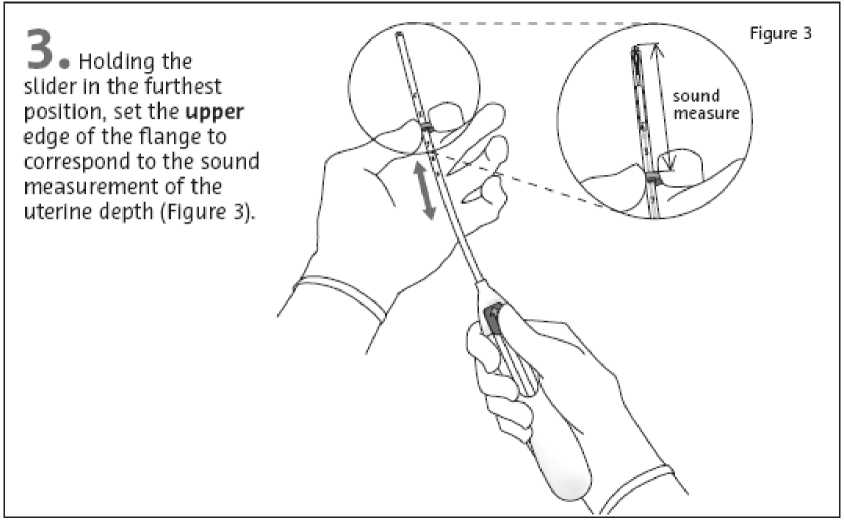
While holding the slider In the furthest position, advance the Inserter through the cervix until the flange Is approx, 1,5-2,0 cm from the uterine cervix [Figure 4),
.L* First, open the sterile package completely [Figure 1), Then use sterile technique and sterile gloves,
IMPORTANT! Do not force the Inserter. Dilate the cervical cana If necessary.
Insertion tube with plunger and scale
Ma rk
Handlewith threads inside
5 * While holding the
Inserter steady, pull the slider to the mark to
open the horizontal arms of Mirena (Figure 5J. Walt 5-10 seconds for the horizontal arms to open completely.
Figure 2
Push the
slider forward In
the direction of
the arrow to the
furthest position
to oad Mirena Into
the
nsertlon tube
(Figure 2).
IMPORTANT!
Do not pull the
slider downwards as
this may prematurely
release Mirena, Once released,
Mirena cannot be re-Loaded,
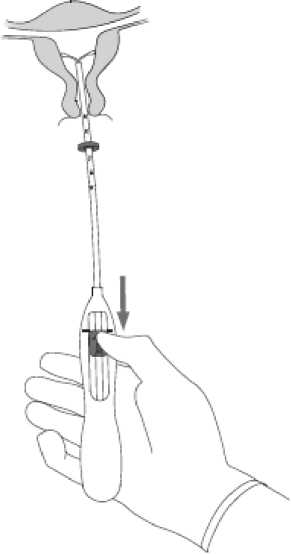
Figure 5
6 • Advance the Inserter gently towards the fundus of the uterus until the flange touches the cervix. Mlrena Is now In thefundal position [Figure G).
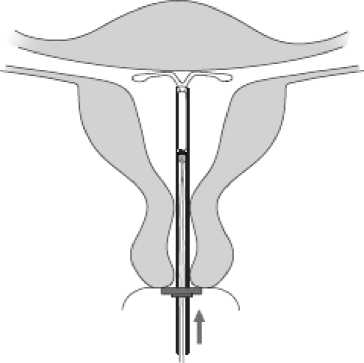
Figure 6
7
Figure 7
Holding the Inserter
In place, release Mlrena
by pulling the slider all
the way down (Figure 7)
While holding the slider
all the way down, gently
remove the Inserter by
pulling It out. Cut the
threads to leave about
2-1 cm visible outside of
the cervix.
IMPORTANT! Should you suspect that the system is not in the correct position, check placement (e.g. with ultrasound). Remove the system if it is not positioned properly within the uterine cavity. A removed system must not be re-inserted.
Removal/replacement
For removal/replacement, please consult the Mirena prescribing information.
Mlrena is removed by
Figure 8
pulling on the threads with
a forceps (Figure S).
You may Insert a new
Mlrena Immediately
following removal
Manufactured by: BAYER OY, PANSIONTIE 47, 20210 TURKU, FINLAND.
PL 20774/1425 30th August 2016 PP2/1425/Insert/V1
This frequency is based on clinical trials that excluded breastfeeding women. In a large prospective comparative non-interventional cohort study in IUD users, the frequency of perforation in women who were breastfeeding or had an insertion up to 36 weeks after delivery was “uncommon” (see section 4.4).