Mirtazapine 30Mg Orodispersible Tablets


Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
PACKAGE LEAFLET: INFORMATION FOR THE USER
MIRTAZAPINE 15 mg ORODISPERSIBLE TABLETS MIRTAZAPINE 30 mg ORODISPERSIBLE TABLETS MIRTAZAPINE 45 mg ORODISPERSIBLE TABLETS
(mirtazapine)
What is in this leaflet:
1. What Mirtazapine is and what it is used for.
2. What you need to know before you take Mirtazapine.
3. How to take Mirtazapine.
4. Possible side effects.
5. How to store Mirtazapine.
6. Contents of the pack and other information.
1. WHAT MIRTAZAPINE IS AND WHAT IT IS USED FOR
Mirtazapine is one of a group of medicines called antidepressants.
Mirtazapine is used to treat depressive illness.
2. WHAT YOU NEED TO KNOW BEFORE YOU TAKE MIRTAZAPINE Do not take Mirtazapine:
• if you are allergic (hypersensitive) to mirtazapine or any of the other ingredients of Mirtazapine. If so, you must talk to your doctor as soon as you can before taking Mirtazapine.
• if you are taking or have recently taken (within the last two weeks) medicines called monoamine oxidase inhibitors (MAO-Is).
Warning and precautions
Talk to you doctor or pharmacist before taking Mirtazapine.
Children and adolescents
Mirtazapine should normally not be used for children and adolescents under 18 years because efficacy was not demonstrated. Also, you should know that patients under 18 have an increased risk of side effects such as suicide attempt, suicidal thoughts and hostility (predominately aggression, oppositional behaviour and anger) when they take this class of medicine. Despite this, your doctor may prescribe Mirtazapine for patients under 18 because he/she decides that this is in their best interests. If the doctor has prescribed Mirtazapine for a patient under 18 and you want to discuss this, please go back to your doctor. You should inform your doctor if any of the symptoms listed above develop or worsen when patients under 18 are taking Mirtazapine. Also the long-term safety effects concerning growth, maturation and cognitive and behavioural development of Mirtazapine in this age group have not yet been demonstrated. In addition, significant weight gain has been observed in the age category more often when treated with Mirtazapine compared with adults.
Thoughts of suicide and worsening of your depression
If you are depressed you can sometimes have thoughts of harming or killing yourself. These may be increased when first starting antidepressants, since these medicines all take time to work, usually about two weeks but sometimes longer.
You may be more likely to think like this:
• if you have previously had thoughts about killing or harming yourself
• if you are a young adult. Information from clinical trials has shown an increased risk of suicidal behaviour in adults aged less than 25 years with psychiatric conditions who were treated with an antidepressant
O If you have thoughts of harming or killing yourself at any time, contact your doctor or go to a hospital straight away.
You may find it helpful to tell a relative or close friend that you are depressed, and ask them to read this leaflet. You might ask them to tell you if they think your depression is getting worse, or if they are worried about changes in your behaviour.
Also take special care with Mirtazapine:
• if you have, or have ever had one of the following conditions
O Tell your doctor about these conditions before taking Mirtazapine, if not done previously:
* seizures (epilepsy). If you develop seizures or your seizures become more frequent, stop taking Mirtazapine and contact your doctor immediately
* liver disease, including jaundice. If jaundice occurs, stop taking Mirtazapine and contact your doctor immediately
* kidney disease
* heart disease, or low blood pressure
* schizophrenia. If psychotic symptoms, such as paranoid thoughts become more frequent or severe, contact you doctor straight away
* manic depression (alternating periods of feeling elated/overactivity and depressed mood). If you start feeling elated or over-excited, stop taking Mirtazapine and contact your doctor immediately
* diabetes (you may need to adjust your dose of insulin or other antidiabetic medicines)
* eye disease, such as increased pressure in the eye (glaucoma)
* difficulty in passing water (urinating), which might be caused by an enlarged prostate.
• if you develop signs of infection such as inexplicable high fever, sore throat and mouth ulcers O Stop taking Mirtazapine and consult your doctor immediately for a blood test.
In rare cases these symptoms can be a sign of disturbances in blood cell production in the bone marrow. While rare, these symptoms most commonly appear after 4-6 weeks of treatment
• if you are an elderly person. You could be more sensitive to the side-effects of antidepressants.
Other medicines and Mirtazapine
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Do not take Mirtazapine in combination with:
• monoamine oxidase inhibitors (MAO inhibitors). Also, do not take Mirtazapine during the two weeks after you have stopped taking MAO inhibitors. If you stop taking Mirtazapine, do not take MAO inhibitors during the next two weeks either.
Examples of MAO inhibitors are moclobemide, tranylcypromine (both are antidepressants) and selegiline (used for Parkinson's disease).
Take care when taking Mirtazapine in combination with:
• antidepressants such as SSRIs, venlafaxine and L-tryptophan, or triptans (used to treat migraine), tramadol (a pain-killer), linezolid (an antibiotic), lithium (used to treat some psychiatric conditions) and St. John's Wort - Hypericum perforatum preparations (a herbal remedy for depression). In very rare cases Mirtazapine alone or the combination of Mirtazapine with these medicines, can lead to a so-called serotonin syndrome. Some of the symptoms of this syndrome are: inexplicable fever, sweating, increased heart rate, diarrhoea, (uncontrollable) muscle contractions, shivering, overactive reflexes, restlessness, mood changes, and unconsciousness. If you get a combination of these symptoms, talk to your doctor immediately
• the antidepressant nefazodone. It can increase the amount of Mirtazapine in your blood. Inform your doctor if you are using this medicine. It might be needed to lower the dose of Mirtazapine, or when use of nefazodone is stopped, to increase the dose of Mirtazapine again
• medicines for anxiety or insomnia such as benzodiazepines; medicines for schizophrenia such as olanzapine; medicines for allergies such as cetirizine;
medicines for severe pain such as morphine.
In combination with these medicines Mirtazapine can increase the drowsiness caused by these medicines
• medicines for infections; medicines for bacterial infections (such as erythromycin); medicines for fungal infections (such as ketoconazole) and medicines for HIV/AIDS (such as HIV- protease inhibitors).
In combination with Mirtazapine these medicines can increase the amount of Mirtazapine in your blood. Inform your doctor if you are using these medicines. It might be needed to lower the dose of Mirtazapine, or when these medicines are stopped, to increase the dose of Mirtazapine again
• medicines for epilepsy such as carbamazepine and phenytoin; medicines for tuberculosis such as rifampicin.
In combination with Mirtazapine these medicines can reduce the amount of Mirtazapine in your blood. Inform your doctor if you are using these medicines. It might be needed to increase the dose of Mirtazapine, or when these medicines are stopped to lower the dose of Mirtazapine again
• medicines to prevent blood clotting such as warfarin.
Mirtazapine can increase the effects of warfarin on the blood. Inform your doctor if you are using this medicine. In case of combination it is advised that a doctor monitors your blood carefully
• Medicines for stomach ulcers, heartburn or gastroesophageal reflux disease (GERD) such as cimetidine can increase the amount of Mirtazapine in your blood. Inform your doctor if you are using this medicine as your doctor may need to lower your dose of Mirtazapine. When use of cimetidine is stopped, your doctor may need to increase your dose of Mirtazapine again.
Mirtazapine with food, drink and alcohol
You may get drowsy if you drink alcohol while you are taking Mirtazapine.
You are advised not to drink any alcohol.
You can take Mirtazapine with or without food.
Pregnancy, breast-feeding and fertility
Make sure your midwife and/or doctor knows you are on Mirtazapine.
Limited experience with Mirtazapine administration to pregnant women does not indicate an increased risk. However, caution should be exercised when used during pregnancy.
If you are taking Mirtazapine and you become pregnant or you plan to get pregnant, ask your doctor whether you may continue taking Mirtazapine. If you use Mirtazapine until, or shortly before birth, your baby should be supervised for possible adverse effects.
When taken during pregnancy, similar drugs (SSRIs) may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the newborn (PPHN), making the baby breathe faster and appear bluish. These symptoms usually begin during the first 24 hours after the baby is born. If this happens to your baby you should contact your midwife and/or doctor immediately.
Ask your doctor whether you can breast-feed, while taking Mirtazapine.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Mirtazapine can affect your concentration or alertness. Make sure these abilities are not affected before you drive or operate machinery.
Mirtazapine contains Aspartame
Mirtazapine contain aspartame, a source of phenylalanine. May be harmful for people with phenylketonuria.
3. HOW TO TAKE MIRTAZAPINE
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
How much to take
The recommended dose is 15 or 30 mg every day. Your doctor may advise you to increase your dose after a few days to the amount that is best for you (between 15 and 45 mg per day). The dose is usually the same for all ages. However, if you are an elderly person or if you have renal or liver disease, your doctor may adapt the dose.
When to take Mirtazapine O Take Mirtazapine at the same time each day.
It is best to take Mirtazapine as a single dose before you go to bed. However your doctor may suggest to split your dose of Mirtazapine - once in the morning and once at night-time before you go to bed. The higher dose should be taken before you go to bed.
Time: 12:50
Date: 19 AUG 2014
|
Description |
Mirtazepine 15/30/45mg ALL |
No. of colours | ||
|
Component Type |
Leaflet |
Pharma Code |
TBC |
Colours |
|
Affiliate Item Code |
378162 |
SAP No. |
N/A | |
|
Superceded Affiliate Item Code |
10005290 |
Vendor Job No. |
224120 |
Non-Print Colours |
|
TrackWise PR No. |
378162 |
Proof No. |
7 |
Equate CMYK |
|
MA No. |
04569/0794, 0795 & 0796 |
Client Market |
UK |
with |
|
Packing Site/Printer |
N/A |
Keyline/DrawingNo] |
N/A |
Main Font |
|
Supplier Code |
TBC |
Barcode Info |
N/A |
Dimensions |
|
Sign-offs | ||||
Page Count
1/2
Myriad Pro
Body Text Size
8 pt
280 x 480mm
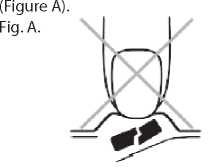
Take the orodispersible tablet as follows
Take your tablets orally.
1. Do not crush the orodispersible tablet
In order to prevent crushing the orodispersible tablet, do not push against the tablet pocket
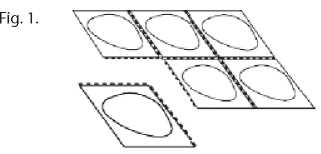
2. Tear off one tablet pocket
Each blister contains tablet pockets, which are separated by perforations. Tear off one tablet pocket along the

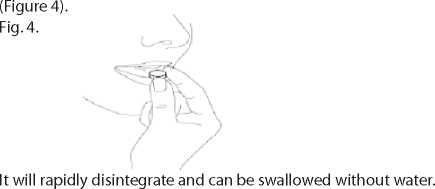
4. Take out the orodispersible tablet
Take out the orodispersible tablet with dry hands and place it on the tongue.
When can you expect to start feeling better
Usually Mirtazapine will start working after 1 to 2 weeks and after 2 to 4 weeks you may start to feel better.
It is important that, during the first few weeks of the treatment, you talk with your doctor about the effects of Mirtazapine:
O 2 to 4 weeks after you have started taking Mirtazapine, talk to your doctor about how this medicine has affected you.
If you still don't feel better, your doctor may prescribe a higher dose. In that case, talk to your doctor again after another 2 to 4 weeks. Usually you will need to take Mirtazapine until your symptoms of depression have disappeared for 4 to 6 months.
If you take more Mirtazapine than you should:
O If you or someone else have taken too much Mirtazapine, call a doctor straight away.
The most likely signs of an overdose of Mirtazapine (without other medicines or alcohol) are drowsiness, disorientation and increased heart rate.
If you forget to take Mirtazapine
If you are supposed to take your dose once a day:
• If you have forgotten to take your dose of Mirtazapine, do not take the missed dose. Just skip it.
Take your next dose at the normal time.
If you are supposed to take your dose twice a day:
• if you have forgotten to take your morning dose, simply take it together with your evening dose
• if you have forgotten to take your evening dose, do not take it with the next morning dose; just skip it and continue with your normal morning and evening doses
• if you have forgotten to take both doses, do not attempt to make up for the missed doses. Skip both doses and continue the next day with your normal morning and evening doses.
If you stop taking Mirtazapine:
O Only stop taking Mirtazapine in consultation with your doctor.
If you stop too early, your depression might come back. Once you are feeling better, talk to your doctor. Your doctor will decide when treatment can be stopped.
Do not suddenly stop taking Mirtazapine, even when your depression has lifted. If you suddenly stop taking Mirtazapine you may feel sick, dizzy, agitated or anxious, and have headaches. These symptoms can be avoided by stopping gradually. Your doctor will tell you how to decrease the dose gradually.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following happen, do not take more Mirtazapine. Tell your doctor immediately or go to the casualty department at your nearest hospital:
• epileptic attack (convulsions)
• yellow colouring of eyes or skin; this may suggest disturbance in liver function (jaundice)
• a combination of symptoms such as inexplicable fever, sweating, increased heart rate, diarrhoea, (uncontrollable) muscle contractions, shivering, overactive reflexes, restlessness, mood changes and unconsciousness. In very rare cases these can be signs of serotonin syndrome.
• thoughts of harming or killing yourself
• Fever, followed by a widespread rash with blisters and peeling skin, particularly around the mouth, nose, eyes and genitals (Stevens Johnson syndrome)
• a widespread rash with blisters and skin peeling on much of the body surface (toxic epidermal necrolysis)
• feeling elated or emotionally 'high' (mania)
• Mirtazapine can cause disturbances in the production of blood cells (bone marrow depression). Some people become less resistant to infection because Mirtazapine can cause a temporary shortage of white blood cells (granulocytopenia). In rare cases Mirtazapine can also cause a shortage of red and white blood cells, as well as blood platelets (aplastic anemia), a shortage of blood platelets (thrombocytopenia) or an increase in the number of white blood cells (eosinophilia).
The following events are less serious, but you may wish to discuss them with your doctor or pharmacist: Very common: may affect more than 1 in 10 people:
• increase in appetite and weight gain • drowsiness or sleepiness • headache
• dry mouth.
Common: may affect up to 1 in 10 people:
• lethargy • dizziness • shakiness or tremor
• nausea • diarrhoea • vomiting
• rash or skin eruptions (exanthema) • pain in your joints (arthralgia) or muscles (myalgia)
• back pain • feeling dizzy or faint when you stand up suddenly (orthostatic
hypotension)
• swelling (typically in ankles or feet) caused by fluid retention (oedema) • tiredness
• vivid dreams • confusion • feeling anxious
• sleeping problems.
In children under 18 years the following adverse events were observed commonly in clinical trials: significant weight gain, hives and increased blood triglycerides.
Uncommon: may affect up to 1 in 100 people:
• abnormal sensation in the skin e.g. burning, stinging, tickling or tingling (paraesthesia)
• restless legs • fainting (syncope)
• sensations of numbness in the mouth (oral hypoaesthesia) • low blood pressure
• nightmares • feeling agitated • hallucinations
• urge to move.
Rare: may affect up to 1 in 1,000 people:
• muscle twitching or contractions (myoclonus)
• aggressive behaviour
• inflammation of the pancreas. This causes moderate to severe pain in the stomach.
Not known: frequency cannot be estimated from the available data:
• Fever, followed by a skin rash which may look like a blister, and looks like small targets (central dark spots surrounded by a paler area, with a dark ring around the edge) particularly on the face and extremities called erythema multiforme
• abnormal sensations in the mouth (oral paraesthesia)
• swelling in the mouth (mouth oedema)
• hyponatraemia
• inappropriate anti-diuretic hormone secretion
• increased salivation
• skin rash with blisters (bullous dermatitis)
• sleepwalking
• difficulty in speaking
Reporting side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE MIRTAZAPINE
Keep this medicine out of the sight and reach of children.
Do not use Mirtazapine after the expiry date which is stated on the carton and the blister. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Do not throw away medicines via wastewater or household waste.
Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Mirtazapine contains
• The active substance is mirtazapine.
Mirtazapine 15 mg orodispersible tablets contain 15 mg mirtazapine per orodispersible tablet.
Mirtazapine 30 mg orodispersible tablets contain 30 mg mirtazapine per orodispersible tablet.
Mirtazapine 45 mg orodispersible tablets contain 45 mg mirtazapine per orodispersible tablet.
• The other ingredients are:
crospovidone, mannitol [E421], microcrystalline cellulose, aspartame (E951), strawberry guarana flavour, peppermint flavour, colloidal anhydrous silica and magnesium stearate.
What Mirtazapine looks like and contents of the pack
Mirtazapine are orodispersible tablets.
Mirtazapine 15 mg orodispersible tablets are round, white tablets marked with 'A' on one side and marked '36' on the other side.
Mirtazapine 30 mg orodispersible tablets are round, white tablets marked with 'A' on one side and marked '37' on the other side.
Mirtazapine 45 mg orodispersible tablets are round, white tablets marked with 'A' on one side and marked '38' on the other side.
For Mirtazapine 15, 30 and 45 mg orodispersible tablets the following pack sizes are available: 6, 12, 18, 30, 48, 60, 90,96 and 100 orodispersible tablets (not all pack sizes may be marketed)
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Mylan, Potters Bar, Herts., EN6 1TL, United Kingdom.
Manufacturer:
Gerard Laboratories, 35/36 Baldoyle Industrial Estate, Grange Road, Dublin 13, Ireland.
Generics [UK] Ltd., Potters Bar, Herts., EN6 1TL, United Kingdom.
Mylan Hungary Kft, H-2900 Komarom, Mylan utca 1, Hungary
378162
|
Description |
Mirtazepine 15/30/45mg ALL |
No. of colours | ||
|
Component Type |
Leaflet |
Pharma Code |
TBC |
Colours |
|
Affiliate Item Code |
378162 |
SAP No. |
N/A | |
|
Superceded Affiliate Item Code |
10005290 |
Vendor Job No. |
224120 |
Non-Print Colours |
|
TrackWise PR No. |
378162 |
Proof No. |
7 |
Equate CMYK |
|
MA No. |
04569/0794, 0795 & 0796 |
Client Market |
UK |
with |
|
Packing Site/Printer |
N/A |
Keyline/DrawingNo] |
N/A |
Main Font |
|
Supplier Code |
TBC |
Barcode Info |
N/A |
Dimensions |
|
Sign-offs | ||||
Page Count
2/2
Myriad Pro
Body Text Size
8 pt
280 x 480mm