Mitocin 20 Mg Powder And Solvent For Intravesical Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Mitocin 20 mg, powder and solvent for intravesical solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active substance: Mitomycin
1 vial of Mitocin powder for intravesical solution contains 20 mg mitomycin. After reconstitution with the solvent included 1 ml of intravesical solution contains 1 mg mitomycin.
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Powder and solvent for intravesical solution
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Intravesical administration for relapse prevention in superficial urinary bladder carcinoma after transurethral resection.
4.2 Posology and method of administration
Posology
Intravesical administration
In intravesical therapy, 20 - 40 mg of mitomycin, corresponding to 1 - 2 vials of Mitocin20 mg in 20 - 40 ml of sodium chloride 9 mg/ml (0.9%) solution (for intravesical use), is instilled weekly into the bladder. In the case of intravesical administration the urine pH should be higher than pH 6.
Alternative dose recommendation in the prevention of recurrent superficial bladder tumours is 4-10 mg (0.06-0.15 mg/kg of body weight) instilled into the bladder though a urethral catheter 1 or 3 times per week
Special population
The dose must be reduced in patients who have undergone extensive previous cytostatic therapy, in case of myelosuppression or in elderly patients.
Insufficient data from clinical studies are available concerning the use of mitomycin in patients >65 years of age.
The product should not be used in patients with renal impairment (see section 4.3)
The product is not recommended in patients with hepatic impairment due to lack efficacy and safety data in this group of patients.
Paediatric population
The safety and efficacy of mitomycin in children have not been established.
Method of administration
Mitomycin is intended for intravesical instillation after being dissolved. Partial use is applicable.
Preparation of ready-to-use solution for intravesical administration
The contents of 1 - 2 vials of Mitocin 20 mg (equivalent to 20 - 40 mg of mitomycin) are dissolved in 20 - 40 ml of sodium chloride 9 mg/ml (0.9%) solution (for intravesical use).
For use of the instillation set of Mitocin 20 mg the corresponding instructions for use must be followed. The 0.9% sodium chloride solution in the bag is used for preparation of the reconstituted solution.
4.3
Contraindications
• Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
• Breastfeeding
Intravesical therapy
Perforation of the bladder wall is an absolute contraindication.
Cystitis is a relative contraindication.
4.4 Special warnings and precautions for use
Due to the toxic effects on the bone marrow of mitomycin, other myelotoxic therapy modalities (in particular other cytostatics, radiation) must be administered with particular caution in order to minimise the risk of additive myelosuppression.
Long-term therapy may result in cumulative bone marrow toxicity. Bone marrow suppression may only manifest itself after a delay, being expressed most strongly after 4 - 6 weeks, accumulating after prolonged use and therefore often requiring an individual dose adjustment.
Elderly patients often have reduced physiological function, bone marrow depression, which may be protracted, so administer mitomycin with special caution in this population while closely monitoring patient's condition.
Mitomycin is a mutagenic and potentially carcinogenic substance in humans. Contact with the skin and mucous membranes is to be avoided.
In the case of pulmonary symptoms, which cannot be attributed to the underlying disease, therapy should be stopped immediately. Pulmonary toxicity can be well treated with steroids.
Therapy should be stopped immediately also if there are symptoms of haemolysis or indications of renal dysfunction (nephrotoxicity).
At doses of > 30 mg of mitomycin/m2 of body surface microangiopathic-haemolytic anaemia has been observed. Close monitoring of renal function is recommended.
New findings suggest a therapeutic trial may be appropriate for the removal of immune complexes that seem to play a significant role in the onset of symptoms by means of staphylococcal protein A.
Occurrence of acute leukaemia (in some cases following preleukaemic phase) and myelodysplastic syndrome has been reported in the patients treated concomitantly with other antineoplastic agents.
The medicinal product Mitocin20 mg after reconstitution in the bag containing the solvent contains 3.08 mmol (70.8 mg) of sodium per 20 ml of solution. This should be taken into consideration by patients on a controlled sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction
Myelotoxic interactions with other bone marrow-toxic treatment modalities (especially other cytotoxic medicinal products, radiation) are possible.
Combination with vinca alkaloids or bleomycin may reinforce pulmonary toxicity.
An increased risk of haemolytic-uremic syndrome has been reported in patients receiving a concomitant administration of mitomycin and fluorouracil or tamoxifen.
In animal experiments, pyridoxine hydrochloride (vitamin B6) resulted in the loss of effect of mitomycin.
No injections with live vaccines should be carried out in connection with mitomycin treatment.
The cardiotoxicity of Adriamycin (doxorubicin) may be reinforced by mitomycin.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no data from the use of mitomycin in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3). Mitomycin has a mutagenic, teratogenic and carcinogenic effect and therefore may impair the development of an embryo. Mitomycin should not be used during pregnancy. In the case of a vital indication for the treatment of a pregnant patient a medical consultation should be carried out with respect to the risk of the harmful effects on the child, which are associated with the treatment.
Breastfeeding
It is suggested that mitomycin is excreted in breast milk. Due to its proven mutagenic, teratogenic and carcinogenic effects, mitomycin may not be administered during breastfeeding and therefore Mitocin is contraindicated during breastfeeding (see section 4.3).
Fertility/ Contraception in males and females
Female patients of a sexually mature age should take contraceptive measures during and up to 6 months after the end of chemotherapy or refrain from sexual intercourse.
Mitomycin has a genetically harmful effect. Men who are being treated with mitomycin are therefore advised not to father a child during treatment and up to 6 months thereafter and to seek advice on the preservation of sperm before the start of therapy due to the possibility of irreversible infertility caused by the therapy with mitomycin.
4.7 Effects on ability to drive and use machines
Even when used in accordance with instructions these medicinal products may cause nausea and vomiting and thereby reduce reaction times to such an extent that the ability to drive a motor vehicle or operate machinery is impaired. This applies even more in connection with alcohol.
4.8 Undesirable effects
Undesirable effects are listed below by system organ class and frequency.
Frequencies below are defined as:
Very common (> 1/10), comment (> 1/100 to < 1/10), uncommon (> 1/1,000 to < 1/100), rare (> 1/10,000 to < 1/1,000), very rare (< 1/10,000) or not known (cannot be estimated from the available data)
Possible side-effects under systemic therapy
The most common side effects of mitomycin administered systemically are gastrointestinal symptoms like nausea and vomiting and bone marrow suppression with leukopenia and mostly dominant thrombocytopenia. This bone marrow suppression occurs in up to 65% of patients.
In up to 10% of patients serious organ toxicity in the form of interstitial pneumonia or nephrotoxicity must be expected.
Mitomycin is potentially hepatotoxic.
|
Blood and the lymphatic system disorders |
Very common Bone marrow suppression, leucopenia thrombocytopenia Rare Life-threatening infection, sepsis, haemolytic anaemia |
|
Immune system disorders |
Very rare Severe allergic reaction |
|
Cardiac disorders |
Rare Heart failure after previous therapy with anthracyclines |
|
Respiratory, thoracic and mediastinal disorders |
Common Interstitial pneumonia,dyspnoe, cough, shortness of breath Rare Pulmonary hypertension, pulmonary veno-occlusive disease (PVOD) |
|
Gastrointestinal disorders |
Very common Nausea, vomiting, Uncommon Mucositis, stomatitis, diarrhoea, anorexia |
|
Hepato-biliary disorders |
Rare Liver dysfunction, increased transaminases, jaundice, veno-occlusive disease (VOD) of the liver |
|
Skin and subcutaneous tissue disorders |
Common Exanthema, allergic skin rash, contact dermatitis, palmar-plantar erythema Uncommon Alopecia Rare Generalised exanthema |
|
Renal and urinary disorders |
Common Renal dysfunction, increase in serum creatinine, glomerulopathy, Nephrotoxicity |
|
Rare Haemolytic uraemic syndrome (HUS) (commonly fatal), microangiopathic-haemolytic anaemia (MAHA syndrome) | |
|
General disorders and administration site conditions |
Common Following Extravasation: Cellulitis, tissue necrosis Uncommon Fever |
Possible side-effects under intravesical therapy
|
Skin and subcutaneous tissue disorders |
Common Pruritus, allergic skin rash, contact dermatitis, palmar-plantar erythema Rare Generalised exanthema |
|
Renal and urinary disorders |
Common Cystitis (possibly haemorrhagic), dysuria, nocturia, pollakisuria, hematuria, local irritation of the bladder wall |
|
Very rare | |
|
necrotizing cystitis, allergic (eosinophilic) cystitis, stenosis of the efferent urinary tract, reduction in bladder capacity, bladder wall calcification and bladder wall fibrosis. |
4.9 Overdose
In case of overdose severe myelotoxicity or even myelophthisis must be expected, with the full-blown clinical effect only appearing after approximately 2 weeks.
The period until which the number of leucocytes falls to the lowest value may be 4 weeks. Prolonged close haematological monitoring therefore also has to be carried out if an overdose is suspected.
As there are no effective antidotes available, the greatest level of caution is required during each application.
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agent, Other cytotoxic antibiotics ATC code: L01DC03
The antibiotic mitomycin is a cytostatic medicinal product from the group of alkylating agents.
Mitomycin is an antibiotic isolated from Streptomyces caespitosus with antineoplastic effect. It is present in an inactive form. Activation to a trifunctional alkylating agent is rapid, either at physiological pH in the presence of NADPH in serum or intracellularly in virtually all cells of the body with the exception of the cerebrum, as the blood-brain barrier is not overcome by mitomycin. The 3 alkylating radicals all stem from a quinone, an aziridine and a urethane group. The mechanism of action is based predominantly on the alkylation of DNA (RNA to a lesser extent) with the corresponding inhibition of DNA synthesis. The degree of DNA damage correlates with the clinical effect and is lower in resistant cells than in sensitive ones. As with other alkylating agents, proliferating cells are damaged to a greater extent than those that are in the resting phase (GO) of the cell cycle. Additionally, free peroxide radicals are released, particularly in the case of higher doses, which result in DNA breaks. The release of peroxide radicals is associated with the organ-specific pattern of side-effects.
5.2 Pharmacokinetic properties
After the intravenous administration of 10 - 20 mg/m2 of mitomycin, maximum plasma levels of 0.4 - 3.2 pg/ml have been measured. The biological half-life is short and is between 40 and 50 minutes. The serum level falls biexponentially, steeply at first within the first 45 minutes, and then more slowly.
After approximately 3 hours the serum levels are usually below the detection limit. The main location for metabolism and elimination is the liver. Accordingly, high concentrations of mitomycin have been found in the gall bladder. Renal excretion plays only a minor role with respect to the elimination.
During intravesical therapy mitomycin is only absorbed in insignificant doses. Nevertheless, a systemic effect cannot be excluded completely.
5.3 Preclinical safety data
In animals, mitomycin is toxic to all proliferating tissues, particularly the cells of the bone marrow and the mucous membrane of the gastrointestinal tract, resulting in the inhibition of spermiogenesis.
Mitomycin has mutagenic, carcinogenic and teratogenic effects which can be demonstrated in corresponding experimental systems.
Local tolerance
Mitomycin causes severe necrosis in the case of paravenous injection or leakage from the blood vessel into surrounding tissue.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Mannitol (Ph.Eur.),
36% hydrochloric acid and sodium hydroxide for pH adjustment, Solvent for intravesical solution: sodium chloride and water for injections.
6.2 Incompatibilities
Incompatibilities occur with highly acidic or alkaline substances. The optimum pH of the ready-to-use mitomycin solution is 7.0.
6.3 Shelf life
Instillation Set: 1 year
Reconstituted solution:
Only clear solutions may be used.
The contents of the vials are intended for single use only. Unused solutions must be discarded.
The chemical and physical stability at room temperature and during exposure to light of a reconstituted solution is
• 2 hours with sodium chloride 9 mg/ml (0.9%) solution (for intravesical use)
All reconstituted solutions are intended for immediate use!
6.4 Special precautions for storage
Do not store above 25°C. Store the vial in the outer carton in order to protect the contents from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5 Nature and contents of container
Packs of 1 amber glass vial (type I), 1 PVC bag of 20 ml with sodium chloride 9 mg/ml (0.9%) solution (for intravesical use), 1 Tiemann catheter
Packs of 4 amber glass vials (type I), 4 PVC bags of 20 ml with sodium chloride 9 mg/ml (0.9%) solution (for intravesical use), 4 Tiemann catheters
Packs of 5 amber glass vials (type I), 5 PVC bags of 20 ml with sodium chloride 9 mg/ml (0.9%) solution (for intravesical use), 5 Tiemann catheters
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Special precautions for the preparation and the disposal of unused cytotoxic medicinal products should be complied with.
The reconstituted solution should be stored away from light in the refrigerator.
Before the ready-to-use solution is used it should be warmed up to room or body temperature.
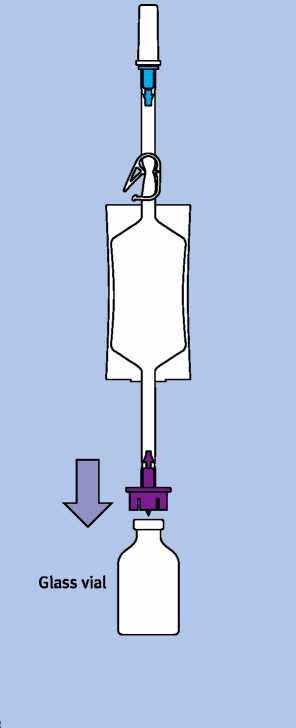
Instructions for use for the solvent for intravesical solution (instillation set):
Remove the bag with the sodium chloride 9 mg/ml (0.9%) solution (for intravesical use) out of the transparent protective film.
The white clip below the bag containing the sodium chloride 9 mg/ml (0.9%) solution (for intravesical use) should be and remain open.
Pull the protective cap off the violet adapter. Ensure that the disposal bag is readily available.

Take the Mitocin vial out of the folding box.
Remove the white cap from the vial.
Place the violet adapter centrally and vertically on the rubber stopper and press the violet adapter into the rubber stopper of the vial until it locks into place.
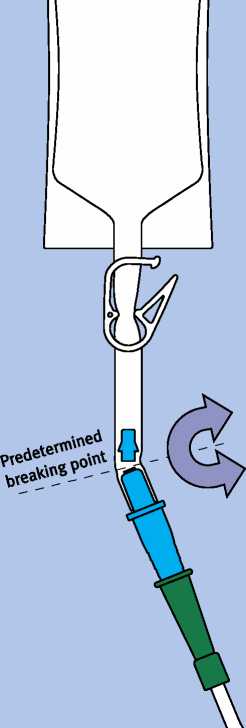
Bend the part of the violet adapter located in the tube at the predetermined breaking point backwards and forwards until the cone breaks off and the connection is open.

Hold the solvent-containing bag with the vial facing downwards.
Squeeze the bag together several times until the solvent has flowed into the vial.

Screw the vial upwards again.
Press the air out of the solvent bag into the vial: if the pressure falls, the mitomycin solution collects in the bag.
Repeat this step once or twice if necessary. It is acceptable for a small residue of the liquid to finally remain in the Mitocin vial.
|
£ |
g |
|
1 t | |
|
r | |
When you have inserted the catheter into the urethra / bladder and would like to begin with the instillation, pull the transparent cap off the blue catheter adapter. Then push the blue catheter adapter firmly into the green attachment of the catheter.
M

Break off the blue catheter adapter in the tube section at the predetermined breaking point in such a way that the mitomycin solution can flow through the catheter into the bladder.
In order to prevent subsequent trickling you can close the clip after instillation.
Please use the disposal bag to dispose of all parts which have come into contact with the mitomycin solution in the special waste.
7 MARKETING AUTHORISATION HOLDER
Speciality European Pharma Limited □
14 Took’s Court, London EC4A 1LB^
Tel: +44 (0)20 7421 7400 □
Fax: +44 (0)20 7421 7401
8 MARKETING AUTHORISATION NUMBER(S)
PL 32828/0003
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
11/06/2013
DATE OF REVISION OF THE TEXT
11/11/2013