Mitomycin 20 Mg Powder And Solvent For Intravesical Solution

Package leaflet: Information for the user
Mitomycin, 20 mg, powder and solvent for intravesical solution
Mitomycin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only.
Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Mitomycin is and what it is used for
2. What you need to know before you use Mitomycin
3. How to use Mitomycin
4. Possible side effects
5. How to store Mitomycin
6. Contents of the pack and other information
1. What Mitomycin is and what it is used for
Mitomycin is a medicine for the treatment of cancer, i.e. a medicine which prevents or considerably delays the division of active cells by influencing their metabolism in various ways. The therapeutic use of cytostatics in cancer therapy is based on the fact that one way in which cancer cells differ from normal cells in the body is that the rate of cell division is increased due to a lack of control of their growth.
Therapeutic indications
Application in the urinary bladder (intravesical application) for the prevention of a relapse in the case of superficial urinary bladder cancer after removal of tissue through the urethra (transurethral resection).
2. What you need to know before you use Mitomycin
Mitomycin may only be administered if strictly indicated, and by doctors experienced in this type of therapy.
Do not use Mitomycin
• if you are allergic to mitomycin or any of the other ingredients of this medicine (listed in section 6),
• while breast-feeding: you must not breast-feed during treatment with mitomycin,
• if you have a perforation of the bladder wall,
• if you suffer from an inflammation of the urinary bladder (cystitis).
Warnings and precautions
Talk to your doctor or pharmacist before using Mitomycin.
Particular caution is required when using Mitomycin
• if you are in poor general health,
• if you are suffering from impaired lung, kidney or liver function,
• if you are undergoing radiation therapy,
• if you are being treated with other cytostatics (substances which inhibit cell growth/cell division),
• if you have been told that you have bone marrow depression (your bone marrow is not able to make the blood cells that you need) it may become worse (especially in older people and during long-term treatment with mitomycin); infection may be aggravated due to bone marrow depression and may lead to fatal conditions, if you are of child-bearing age as mitomycin may affect your ability to have children in the future.
Mitomycin is a substance that can cause significant hereditary changes in genetic material, and can also cause cancer in humans.
Avoid contact with the skin and mucous membranes.
Children and adolescents
The use of mitomycin in children and adolescents is not recommended.
Other medicines and Mitomycin
There are no known interactions of intravesically given mitomycin with other medicines.
Possible interaction under systemic therapy
If other forms of treatment (in particular other anti-cancer medicines, radiation) which also have a harmful effect on the bone marrow are used at the same time, it is possible that the harmful effect of mitomycin on the bone marrow will be intensified.
Combination with vinca alkaloids or bleomycin (medicines belonging to the group of cytostatics) can intensify the harmful effect on the lungs.
An increased risk of a particular form of kidney disease (haemolytic-uraemic syndrome) has been reported in patients receiving a concomitant administration of mitomycin and 5-fluorouracil or tamoxifen.
There are reports from animal experiments that the effect of mitomycin gets lost, if administered together with vitamin B6.
You should not get vaccinated with live vaccines during mitomycin treatment because this may put you at an increased risk to get infected by the live vaccine.
The harmful effect on the heart of Adriamycin (doxorubicin, a medicine belonging to the group of cytostatics) can be intensified by mitomycin.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Mitomycin can cause inherited genetic damage and can adversely affect the development of an embryo.
You must not become pregnant during treatment with mitomycin: If you become pregnant, genetic counselling must be provided.
You should not use mitomycin during pregnancy. If you are pregnant your doctor will decide whether there is a vital indication for treating you with mitomycin and will advise you of the risk of harmful effects on your unborn child associated with the treatment.
Breast-feeding
Mitomycin passes into breast milk. Breast-feeding must be discontinued during treatment.
Fertility / Contraception in males and females As a sexually mature patient you must take contraceptive measures or practise sexual abstinence during chemotherapy and for 6 months afterwards.
Mitomycin can cause inherited genetic damage. As a man treated with mitomycin you are therefore advised not to father a child during treatment and for 6 months afterwards and to seek advice on sperm conservation before starting treatment due to the possibility of irreversible infertility caused by mitomycin therapy.
Driving and using machines
Even when used in accordance with instructions this medicine may cause nausea and vomiting and thereby reduce your reaction times to such an extent that the ability to drive a motor vehicle or operate machinery is impaired. This applies in particular in conjunction with alcohol.
3. How to use Mitomycin
Mitomycin should only be administered by healthcare professionals experienced in this kind of therapy.
Mitomycin is intended to be used for introduction into the urinary bladder (intravesical instillation) after being dissolved.
Your doctor will prescribe a dose that is right for you. If you use more Mitomycin than you should
If you have been accidentally given a higher dose you may experience symptoms such as fever, nausea, vomiting and blood disorders. Your doctor may give you supportive treatment for any symptoms that may occur.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Possible side effects following instillation in the bladder
Common side effects (may affect up to 1 in 10 people):
• bladder inflammation (cystitis) which may be accompanied by blood in the bladder/urine
• painful urination (dysuria)
• frequent urination at night (nocturia)
• excessive frequent urination (pollakiuria)
• blood in the urine (haematuria)
• local irritation of the bladder wall
• localised skin rash (local exanthema)
• allergic skin rash
• skin rash caused by contact with mitomycin (contact dermatitis)
• numbness, swelling and painful redness of palms and soles (palmar-plantar erythema)
Rare (may affect up to 1 in 1,000 people):
• rash over the whole body (generalised exanthema)
Very rare (may affect up to 1 in 10,000 people):
• bladder inflammation with damage of the bladder tissue (necrotising cystitis)
• allergic (eosinophilic) bladder inflammation (cystitis)
• narrowing (stenoses) of the urinary tract
• reduced bladder capacity
• calcium deposits in the bladder wall (bladder wall calcification)
• partial conversion of bladder wall tissue into connective tissue (bladder wall fibrosis)
• decreased number of white blood cells (leukopenia) increasing the risk of infections decreased number of platelets (thrombopenia) causing bruises and bleeding systemic allergic reactions
lung disorder presenting as shortness of breath, dry cough and crackling sounds when breathing in (interstitial lung disease)
increased levels of liver enzymes (transaminases
increased)
hair loss (alopecia)
feeling sick (nausea) and being sick (vomiting) diarrhoea
kidney disease (renal dysfunction) where you pass little or
no urine
fever
Possible side effects following administration into a vein
Severe allergic reaction (symptoms may include faintness, skin rash or hives, itching, swelling of lips, face and airway with difficulty in breathing, loss of consciousness (may affect up to 1 in 10,000 people) may occur.
Severe lung disease presenting as shortness of breath, dry cough and crackling sounds when breathing in (interstitial pneumonia) as well as severe renal dysfunction, kidney disease where you pass little or no urine, etc. may occur.
If you notice any of the above reactions please inform your doctor immediately because mitomycin therapy must be stopped.
Very common side effects
(may affect more than 1 in 10 people):
• inhibition of blood cell production in the bone marrow (bone marrow suppression)
• decreased number of white blood cells (leukopenia) increasing the risk of infections decreased number of platelets (thrombopenia) causing bruises and bleeding
feeling sick (nausea) and being sick (vomiting)
Common side effects (may affect up to 1 in 10 people):
• lung disorder presenting as shortness of breath, dry cough and crackling sounds when breathing in (interstitial pneumonia)
• difficulties breathing (dyspnoea), cough, shortness of breath
• skin rash (exanthema)
• allergic skin rash
• skin rash caused by contact with mitomycin (contact dermatitis)
• numbness, swelling and painful redness of palms and soles (palmar-plantar erythema)
• kidney disorders (renal dysfunction, nephrotoxicity, glomerulopathy, increased levels of creatinine in the blood) where you pass little or no urine
In the event of injection or leakage of mitomycin into the surrounding tissue (extravasation)
• inflammation of connective tissue (cellulitis)
• death of tissue (tissue necrosis)
Uncommon side effects
(may affect up to 1 in 100 people):
• inflammation of the mucous membranes (mucositis)
• inflammation of the mucous membranes in the mouth (stomatitis)
• diarrhoea
• hair loss (alopecia)
• fever
• loss of appetite (anorexia)
Rare (may affect up to 1 in 1,000 people):
• life-threatening infection
• blood poisoning (sepsis)
• decrease in number of red blood cells due to an abnormal breakdown of these cells (haemolytic anaemia)
• heart failure (cardiac insufficiency) after previous therapy with anti-cancer medicines (anthracycline)
• raised blood pressure in the lungs, e.g. leading to shortness of breath, dizziness and fainting (pulmonary hypertension)
• disease involving obstruction of the veins in the lungs (pulmonary veno-occlusive disease, PVOD)
• liver disease (liver dysfunction)
• increased levels of liver enzymes (transaminases)
• yellowing of the skin and whites of the eyes (icterus)
• disease involving obstruction of the veins in the liver (veno-occlusive liver disease, VOD)
• rash over the whole body (generalised exanthema)
• a particular form of kidney failure (haemolytic uraemic syndrome, HUS) characterised by haemolytic anaemia, acute kidney failure, and a low platelet count
• a type of haemolytic anaemia caused by factors in the small blood vessels (microangiopathic haemolytic anaemia, MAHA)
Very rare (may affect up to 1 in 10,000 people):
• severe allergic reaction (symptoms may include faintness, skin rash or hives, itching, swelling of lips, face and airway with difficulty in breathing, loss of consciousness)
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist.
This includes any possible side effects not listed in this
leaflet. You can also report side effects directly via the Yellow
Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more
information on the safety of this medicine.
5. How to store Mitomycin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after “EXP”. The expiry date refers to the last day of that month.
Do not store above 25 °C.
Keep the vial in the outer carton in order to protect from light.
From a chemical and physical point of view the reconstituted product should be used within 24 hours.
From a microbiological point of view, unless the method of opening/reconstitution/dilution precludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of the user.
Protect the reconstituted solution from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Mitomycin contains
• The active substance is mitomycin.
1 vial powder for solution for intravesical use contains 20 mg mitomycin. After reconstitution with 20 ml solvent 1 ml solution for intravesical use contains 1 mg mitomycin.
• The other ingredients are:
Powder for solution for intravesical use:
Urea
Solvent for intravesical solution:
Sodium chloride and water for injections
What Mitomycin looks like and contents of the pack
Mitomycin is a grey to grey-blue powder.
The solvent is a clear and colourless solution.
Mitomycin, 20 mg, powder and solvent for intravesical solution (instillation set) is available in packs with 1,4, 5 or 6 clear glass vials (20 ml) with a coated rubber stopper and aluminium seal. Instillation sets for intravesical instillation also include 1,4, 5 or 6 PVC bags with a volume of 20 ml containing 0.9 % sodium chloride solution and catheters.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
medac Gesellschaft fur klinische Spezialpraparate mbH Theaterstr. 6 22880 Wedel Germany
Tel.: +49 4103 8006-0 Fax: +49 4103 8006-100
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, Hungary, Iceland, Ireland, Latvia, Lithuania, Netherlands, Norway, Poland, Slovak Republic, Sweden: Mitomycin
Germany:
mito-medac
Italy, Portugal: Mitomicina medac
Romania: Mitomicina medac
Slovenia
Mitomicin medac
United Kingdom Mitomycin
This leaflet was last revised in 10/2015.
The following information is intended for healthcare professionals only:
Posology
There are many intravesical mitomycin regimens, varying in dose of mitomycin used, the frequency of instillation and the duration of therapy.
Unless otherwise specified, the dosage of mitomycin is 20 - 40 mg mitomycin instilled into the bladder once weekly. Regimens with instillations every 2 weeks, every month or 3 monthly can also be used.
The specialist should decide on the optimum regime, frequency and duration of therapy on an individual patient basis.
The urine pH should be higher than pH 6.
Reconstitution of the solution for intravesical use ready for use
Instructions for use for the solvent for intravesical solution (instillation set)
Dissolve the content of one vial of Mitomycin (equivalent to 20 mg mitomycin) in 20 ml sterile 0.9 % sodium chloride solution. The contents of the vial must dissolve to form a blue-purple clear solution within 2 minutes.
Only clear solutions may be used.
The content of the vials is intended for single use/single entry only. Unused solution must be discarded.
From a chemical and physical point of view the reconstituted product should be used within 24 hours.
Fig. 1-7:
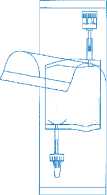
Tear open the protective cover, but do not remove completely! This will keep the tip of the instillation system protected from contamination until the last minute.
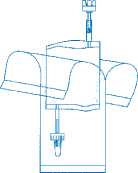
Holding the vial vertically, push it firmly onto the adapter of the instillation system and turn once or twice.
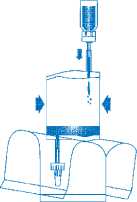
Invert the entire system. Hold the vial up and pump in air. Draw the dissolved mitomycin into the instillation system. Do not remove the vial.
From a microbiological point of view, unless the method of opening/reconstitution/dilution precludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of the user.
Mitomycin must not be used in mixed injections. Other solutions for injection or infusion must be administered separately.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
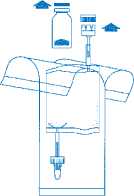
Remove the caps from the vial and the instillation system. Place the disposal bag ready to hand.
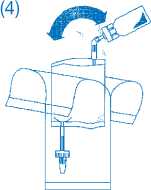
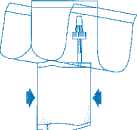
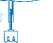
Holding the tube (not the vial) firmly in the vertical position, break the upper valve by bending it backwards and forwards.
Pump the liquid into the vial, but do not fill the vial completely.

Hold the instillation system vertically. Now remove the protective cover completely. Connect the catheter to the system. Now break the sealing mechanism in the tube section by bending backwards and forwards and instil the solution. After completing the instillation, release the catheter by squeezing air through. Keep the solvent bag squeezed together and transfer with the catheter to the disposal bag.
70223-VBGB
AA