Monopost 50 Micrograms/Ml Eye Drops Solution In Single-Dose Container
#
Monopost
50 micrograms/ml
eye drops,
solution in single-dose container
Latanoprost
Read all of this leaflet carefully before
you start using this medicine because it
contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, or pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See Section 4.
What is in this leaflet:
1. What MONOPOST is and what it is used for
2. What you need to know before you use MONOPOST
3. How to use MONOPOST
4. Possible side effects
5. How to store MONOPOST
6. Contents of the pack and other information
l.WHAT MONOPOST IS AND WHAT IT IS USED FOR
MONOPOST belongs to a group of medicines known as prostaglandins. It lowers the pressure within your eye by increasing the natural outflow of fluid from inside the eye into the bloodstream.
MONOPOST is used to treat conditions known as open angle glaucoma and ocular hypertension. Both of these conditions are linked with an increase in the pressure within your eye, eventually affecting your eye sight.
2.WHAT YOU NEED TO KNOW BEFORE YOU USE MONOPOST
Do not use MONOPOST
• If you are allergic (hypersensitive) to latanoprost or any of the other ingredients of this medicine (listed in section 6).
• If you are pregnant or trying to become pregnant.
• If you are breast feeding.
Warnings and precautions
Talk to your doctor, or pharmacist or nurse
before using MONOPOST if you think any of
the following apply to you:
• If you are about to have or have had eye surgery (including cataract surgery).
• If you suffer from eye problems (such as eye pain, irritation or inflammation, blurred vision).
• If you know that you suffer from dry eyes.
• If you have severe asthma or your asthma is not well controlled.
• If you wear contact lenses. You can still use MONOPOST, but follow the instruction for contact lens wearers in Section 3.
• If you have suffered or are currently suffering from a viral infection of the eye caused by the herpes simplex virus (HSV).
Children
MONOPOST has not been investigated in children (below 18 years).
Other medicines and MONOPOST
MONOPOST may interact with other medicines. Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy and breast-feeding Do not use MONOPOST when you are pregnant or breast-feeding.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
When you use MONOPOST you might have blurred vision, for a short time. If this happens to you, do not drive or use any tools or machines until your vision becomes clear again.
Important information about some of the ingredients of MONOPOST
MONOPOST contains macrogolglycerol hydroxystearate (derived from castor oil) which may cause skin reactions.
3.HOW TO USE MONOPOST
Usual dose
• Always use MONOPOST exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
• The usual dose for adults (including the elderly) is one drop once a day in the affected eye(s). The best time to do this is in the evening.
• Do not use MONOPOST more than once a day, because the effectiveness of the treatment can be reduced if you administer it more often.
• Always use MONOPOST exactly as this leaflet tells you or as your doctor has told you, until your doctor tells you to stop. Check with your doctor, or pharmacist or nurse if you are not sure.
Contact lens wearers
If you wear contact lenses, you should remove them before using MONOPOST. After using MONOPOST you should wait 15 minutes before putting your contact lenses back in.
Instructions for use
The drops are supplied in single-dose units.
Use the solution from one single dose unit for administration to the affected eye(s) immediately after opening the container. Since sterility cannot be maintained after opening, throw away the container immediately after using the solution. Open a new single-dose unit each time you use MONOPOST.
These instructions tell you how to use the drops:
1. Wash your hands and sit or stand comfortably.
2. Use your finger to gently pull down the lower eyelid of your affected eye.
3. Place the tip of the single-dose container close to, but not touching your eye.
4. Squeeze the single-dose container gently so that only one drop goes into your eye, then release the lower eyelid.
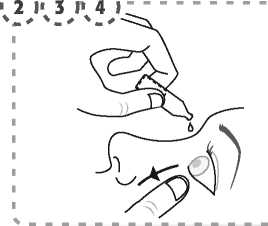
5. Press a finger against the corner of the affected eye by the nose. Hold for 1 minute whilst keeping the eye closed.
\
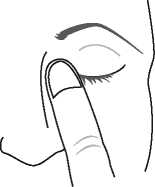
6. Repeat in your other eye if your doctor has told you to do this.
7. Throw away the single-dose container after use. Do not keep it to use it again.
If you use MONOPOST with other eye drops
Wait at least 5 minutes between using MONOPOST and using other eye drops.
I
Monopost
50 micrograms/ml
eye drops,
solution in single-dose container
00000000
00000000

#
If you use more MONOPOST than you should
If you put too many drops into your eye, you may have some minor irritation in your eye and your eyes may water and turn red. This should pass, but if you are worried talk to your doctor.
Contact your doctor as soon as possible if you swallow MONOPOST accidentally.
If you forget to use MONOPOST
Carry on with the usual dosage at the usual time. Do not take a double dose to make up for a forgotten dose. If you are unsure about anything talk to your doctor or pharmacist.
If you stop using MONOPOST
Talk to your doctor if you want to stop using MONOPOST.
If you have any further questions on the use of this medicine, ask your doctor, or pharmacist or nurse.
4.POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common: may affect more than 1 in 10 people
• A gradual change in your eye colour by increasing the amount of brown pigment in the coloured part of the eye known as the iris.
- If you have mixed-colour eyes (blue-brown, grey-brown, yellow-brown or
green-brown) you are more likely to see this change than if you have eyes of one colour (blue, grey, green or brown eyes).
- Any changes in your eye colour may take years to develop although it is normally seen within 8 months of treatment.
- The colour change may be permanent and may be more noticeable if you use MONOPOST in only one eye.
- There appears to be no problems associated with the change in eye colour.
- The eye colour change does not continue after MoNOPOST treatment is stopped.
• Redness of the eye.
• Eye irritation (a feeling of burning, grittiness, itching, stinging or the sensation of a foreign body in the eye).
• A gradual change to eyelashes of the treated eye and the fine hairs around the treated eye, seen mostly in people of Japanese origin. These changes involve an increase of the colour (darkening), length, thickness and number of your eye lashes.
Common: may affect up to 1 in 10 people
• Irritation or disruption to the surface of the eye, eyelid inflammation (blepharitis) and eye pain.
Uncommon: may affect up to 1 in 100 people
• Eyelid swelling, dryness of the eye, inflammation or irritation of the surface of the eye (keratitis), blurred vision and conjunctivitis.
• Skin rash.
Rare: may affect up to 1 in 1,000 people
• Inflammation of the iris, the coloured part of the eye (iritis/uveitis), swelling of the retina (macular oedema), symptoms of swelling or scratching/damage to the surface of the eye, swelling around the eye (periorbital oedema) misdirected eyelashes or an extra row of eyelashes, sensitivity to light (photophobia).
• Skin reactions on the eyelids, darkening of the skin of the eyelids.
• Asthma, worsening of asthma and shortness of breath (dyspnoea).
Very rare: may affect up to 1 in 10,000 people
• Worsening of angina in patients who also have heart disease.
• Chest pain.
• Sunken eye appearance (eye sulcus deepening).
Patients have also reported the following side-effects: fluid filled area within the coloured part of the eye (iris cyst), headache, dizziness, palpitations, muscle pain, joint pain and developing a viral infection of the eye caused by the herpes simplex virus (HSV).
Reporting of side effects
If you get any side effects, talk to your doctor, or pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme.
Website:
By reporting side effects you can help provide more information on the safety of this medicine.
5.HOW TO STORE MONOPOST
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, sachet and single-dose container. The expiry date refers to the last day of that month.
Store below 25°C.
After first opening of the sachet: use the singledose containers within 7 days.
After first opening of the single-dose container, use immediately and throw away the singledose container after use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6.CONTENTS OF THE PACK AND OTHER INFORMATION
What MONOPOST contains
The active substance is latanoprost.
1 ml eye drops solution contains 50 micrograms of latanoprost.
The other ingredients are: macrogolglycerol hydroxystearate 40, sorbitol, carbomer 974P, macrogol 4000, disodium edetate, sodium hydroxide (for pH-adjustment), water for injections.
What MONOPOST looks like and contents of the pack
This medicine is an eye drops, solution in a single-dose container. The solution looks slightly yellow and cloudy. It contains no preservative.
The single-dose containers are packed in a sachet of 5 units, each single-dose container holding 0.2 ml of solution.
A pack size contains 5 (1 sachet of 5),
10 (2 sachets of 5), 3 (6 sachets of 5) or 90 (18 sachets of 5) single-dose containers. Not all pack sizes may be available.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
LABORATOIRES THEA
12, RUE LOUIS BLERIOT
63017 CLERMONT-FERRAND CEDEX 2
FRANCE
Manufacturer
EXCELVISION
27, RUE DE LA LOMBARDIERE ZI LA LOMBARDIERE 07100 ANNONAY FRANCE
This leaflet was last revised in 05/2016.
If you would like any more information, or would like the leaflet in a different format, please contact Medical Information at THEA Pharmaceuticals Ltd, telephone number 0870 192 3283
©Thea
N2345U30ASP/0116