Moviprep Powder For Oral Solution
Package leaflet: Information for the User
MOVIPREP®, powder for oral solution
Macrogol 3350, Sodium sulfate anhydrous, Sodium chloride, Potassium chloride, Ascorbic acid and Sodium ascorbate
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, or pharmacist.
This includes any possible side effects not listed in this leaflet. See section 4.
If you need the information on this leaflet in an alternative format, such as large text, please ring Medical Information, From the UK: 01895 826 606. From Ireland: 00 44 1895 826 606.
What is in this leaflet
1. What Moviprep is and what it is used for
2. What you need to know before you take Moviprep
3. How to take Moviprep
4. Possible side effects
5. How to store Moviprep
6. Contents of the pack and other information
1. What Moviprep is and what it is used for
Moviprep is a lemon flavoured laxative contained in four sachets. There are two large sachets ('sachet A') and two small sachets ('sachet B'). You need all these for one treatment.
Moviprep is intended for adults to clean the bowel so that they are ready for examination.
Moviprep works by emptying the contents of your bowels, so you should expect to have watery bowel movements.
2. What you need to know before you take Moviprep Do not take take Moviprep
• if you are allergic (hypersensitive) to the active substances or any of the other ingredients of this medicine (listed in section 6)
• if you have an obstruction in your intestine (gut)
• if you have a perforated gut wall
• if you have a disorder of stomach emptying
• if you have paralysis of the gut (often occurs after an operation to the abdomen)
• if you suffer from phenylketonuria. This is a hereditary inability of the body to use a particular amino acid. Moviprep contains
a source of phenylalanine
• if your body is unable to produce enough glucose-6-phosphate dehydrogenase
• if you have toxic megacolon (a severe complication of acute colitis)
Warnings and precautions
If you are in poor health or have a serious medical condition, you should be particularly aware of the possible side effects listed in section 4. Contact your doctor or pharmacist if you are concerned.
Talk to your doctor or pharmacist before taking Moviprep if you have any of the following:
• you need to thicken fluids in order to swallow them safely.
• a tendency to regurgitate swallowed drink, food or acid from the stomach.
• kidney disease.
• heart failure or heart disease including high blood pressure, irregular heartbeats or palpitations.
• thyroid disease
• dehydration.
• acute flare of inflammatory bowel disease (Crohn's disease or ulcerative colitis).
Moviprep should not be given to patients with impaired consciousness without medical supervision.
Other medicines and Moviprep
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
If you are taking other medicines take them at least one hour before taking Moviprep or at least one hour afterwards because they may be flushed through your digestive system and not work so well. Moviprep with food and drink
Do not take any solid food from when you start to take Moviprep until after the examination.
When taking Moviprep you should continue to take plenty of fluids. The fluid content of Moviprep does not replace your regular liquid intake.
Pregnancy, breast-feeding and fertility
There are no data on the use of Moviprep during pregnancy or breast feeding and it should only be used if considered essential by the your doctor If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Moviprep does not affect your ability to drive or use machines.
Moviprep contains sodium, potassium and a source of phenylalanine.
This medicinal product contains 56.2 mmol of absorbable sodium per litre. To be taken into consideration by patients on a controlled sodium diet.
This medicinal product contains 14.2 mmol of potassium per litre. To be taken into consideration by patients with reduced kidney function or patients on a controlled potassium diet.
Contains a source of phenylalanine. May be harmful for people with phenylketonuria.
3. How to take Moviprep
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The recommended dose is two litres of solution, which is made up as follows:
This pack contains two clear bags each containing one pair of sachets: sachet A and sachet B. Each pair of sachets (A and B) is to be dissolved in water to make a one litre solution. This pack is therefore sufficient to make up two litres of Moviprep solution.
Use in children
Moviprep should not be taken by children aged below 18 years.
Before you take Moviprep, please read carefully the following instructions. You need to know:
• When to take Moviprep
• How to prepare Moviprep
• How to drink Moviprep
• What you should expect to happen When to take Moviprep
Always take this medicine exactly as described in this leaflet or as your doctor has told you. Check with your doctor if you are not sure. Your treatment with Moviprep must be completed before your clinical procedure:
This course of treatment can be taken either as divided or single doses as described below:
1. Divided doses: one litre of Moviprep in the evening before and one litre in the early morning of the day of the clinical procedure.
2. Single dose: two litres of Moviprep in the evening before the clinical procedure or two litres in the morning of the clinical procedure.
For the divided dose and single dose taken in the evening before the clinical procedure there should be at least one hour between the end of intake of fluid (Moviprep or clear liquid) and the start of the colonoscopy.
For the single dose taken in the morning of the clinical procedure, there should be at least two hours between the end of intake of Moviprep and at least one hour between the end of intake of any clear liquid and the start of the colonoscopy.
Important: Do not take any solid food from when you start to take Moviprep until after the examination.
How to prepare Moviprep
• Open one clear bag and remove the sachets A and B.
• Add the contents of BOTH sachet A and sachet B to a measuring jug that holds 1 litre.
• Add water to make up to the one litre mark of the container and stir until all the powder has dissolved and the Moviprep solution is clear or slightly hazy. This may take up to 5 minutes.
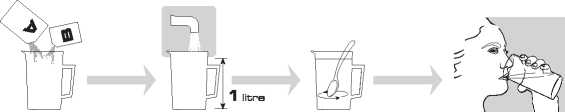
How to drink Moviprep
Drink the first litre of Moviprep solution over one to two hours. Try to drink a glassful every 10 - 15 minutes.
When you are ready, make up and drink the second litre of Moviprep solution made up with the contents of the sachets A and B from the remaining bag.
During the course of this treatment, you are recommended to drink a further one litre of clear liquid to prevent you feeling very thirsty and becoming dehydrated. Water, clear soup, fruit juice (without pulp), soft drinks, tea or coffee (withoutmilk) are all suitable. These drinks can be taken at any time you choose.
What you should expect to happen
When you start drinking the Moviprep solution, it is important that you stay close to a toilet. At some point, you will start to experience watery bowel movements. This is quite normal and indicates that the Moviprep solution is working. The bowel movements will stop soon after you have finished drinking.
If you follow these instructions, your bowel will be clear, and this will help you to have a successful examination. You should allow sufficient time after your last drink to travel to the colonoscopy unit.
If you take more Moviprep than you should If you take more Moviprep than you should you may develop excessive diarrhoea, which can lead to dehydration. Take generous amounts of fluid, especially fruit juices. If you are worried contact your doctor or pharmacist.
If you forget to take Moviprep
If you forget to take Moviprep take the dose as soon as you realise you have not taken it.
If this is several hours after the time when you should have taken it, contact your doctor or pharmacist for advice. It is important that you complete your preparation at least an hour before your clinical procedure when taking Moviprep as divided dose and two hours before your clinical procedure if taking all your Moviprep on the morning of the clinical procedure.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines Moviprep can cause side effects, although not everybody gets them.
It is normal to get diarrhoea when you take Moviprep.
Stop your intake and tell your doctor immediately if you have any of the following side effects:
• rash or itching
• swelling of your face, ankles or other part of your body
• palpitations
• extreme fatigue
• shortness of breath
These are symptoms of a severe allergic reaction.
If you do not have a bowel movement within 6 hours of taking Moviprep, stop the intake and contact your doctor immediately Other side effects include:
Very common side effects (may affect more than 1 in 10 people): Abdominal pain, abdominal distension, tiredness, feeling generally unwell, soreness of the anus, nausea and fever.
Common side effects (may affect up to 1 in 10 people): Hunger, problems sleeping, dizziness, headache, vomiting, indigestion, thirst and chills.
Uncommon side effects (may affect up to 1 in 100 people):
Discomfort, difficulties swallowing, and changes to tests of liver function.
The following side effects have sometimes been seen but it is not known how often they occur because the frequency cannot be estimated from the available data: flatulence (wind), temporary increase in blood pressure, irregular heart rhythm or palpitations, dehydration, retching (straining to vomit), very low blood sodium levels that can cause convulsions (fits) and changes to the levels of salts in the blood such as decreased bicarbonate, increased or decreased calcium; increased or decreased chloride and decreased phosphate. Blood potassium and sodium levels could also decrease. These reactions usually only occur for the duration of the treatment. Should they persist, consult your doctor.
Allergic reactions may cause a skin rash, itching, reddening of the skin or a nettle rash, swollen hands, feet or ankles, headaches, palpitations and shortness of breath.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse.
This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Moviprep
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, after “EXP". Please note that the expiry dates may be different for the different sachets. The expiry date refers to the last day of the month.
Keep Moviprep sachets at room temperature (below 25°C).
After you have dissolved Moviprep in the water, the solution may be stored (keeping covered) at room temperature (below 25°C). It may also be stored in the fridge (2°C -8°C). Do not keep it for more than 24 hours.
|
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment. | |
|
6. Contents of the pack and other information What Moviprep contains Sachet A contains these active substances: | |
|
Macrogol (also known as polyethylene glycol) 3350 |
100 g |
|
Sodium sulfate anhydrous |
7.500 g |
|
Sodium chloride |
2.691 g |
|
Potassium chloride |
1.015 g |
|
Sachet B contains these active substances: | |
|
Ascorbic acid |
4.700 g |
|
Sodium ascorbate |
5.900 g |
The concentration of electrolyte ions when both sachets are made up to one litre of solution is as follows:
|
Sodium |
181.6 mmol/L (of which not more than 56.2 mmol is absorbable) |
|
Chloride |
59.8 mmol/L |
|
Sulfate |
52.8 mmol/L |
|
Potassium |
14.2 mmol/L |
|
Ascorbate |
29.8 mmol/L |
The other ingredients are:
Lemon flavouring (containing maltodextrin, citral, lemon oil, lime oil, xanthan gum, vitamin E), aspartame (E951) and acesulfame potassium (E950) as sweeteners. For further information refer to section 2.
What Moviprep looks like and contents of the pack
This pack contains two clear bags each containing one pair of sachets: sachet A and sachet B. Each pair of sachets (A and B) is to be dissolved in one litre of water.
Moviprep powder for oral solution in sachets is available in pack sizes of 1, 10, 40, 80, 160 and 320 packs of a single treatment. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Norgine BV
Hogehilweg 7, 1101CA Amsterdam ZO,
Manufacturer:
Norgine Limited, New Road, Hengoed,
Mid Glamorgan, CF82 8SJ, United Kingdom. NORGINE
Or
Helsinn Birex Pharmaceuticals Ltd., Damastown Mulhuddart,
Dublin 15, Ireland.
The medicinal product is authorised in the Member States of the EEA under the following names:
Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia,
Finland, France, Germany, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal,
Romania, Slovakia, Slovenia, Spain and United Kingdom: Moviprep.
Sweden: Movprep
This leaflet was last revised in April 2016.
The following information is intended for healthcare professionals only: Moviprep should be administered with caution to fragile patients in poor health or patients with serious clinical impairment such as:
• impaired gag reflex, or with a tendency to aspiration or regurgitation
• impaired consciousness
• severe renal insufficiency (creatinine clearance <30 mL/min)
• cardiac impairment (NYHA grade III or IV)
• those at risk of arrhythmia, for example those on treatment for cardiovascular disease or who have thyroid disease
• dehydration
• severe acute inflammatory disease
The presence of dehydration or electrolyte shifts should be corrected before the use of Moviprep.
Semi-conscious patients or patients prone to aspiration or regurgitation should be closely observed during administration especially if this is via a nasogastric route.
Moviprep should not be given to unconscious patients.
MOVIPREP NORGINE and the sail logo are registered trademarks of the Norgine group of companies.
40601206