Mydrane 0.2 Mg/Ml + 3.1 Mg/Ml + 10 Mg/Ml Solution For Injection.
United Kingdom.................................................................Mydrane
Ireland, Spain........................................................................Fydrane
Norway ...................................................................................Mydane
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT
Mydrane 0.2 mg/mi + 3.1 mg/mi
+ 10 mg/ml solution for injection
tropicamide / phenylephrine hydrochloride / lidocaine hydrochloride
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What MYDRANE is and what it is used for
2. What you need to know before you are given MYDRANE
3. How MYDRANE is administered
4. Possible side effects
5. How to store MYDRANE
6. Contents of the pack and other information
1. WHAT MYDRANE IS AND WHAT IT IS USED FOR What MYDRANE is
This medicine is a solution which is injected into the eye. It contains three active substances:
• tropicamide which belongs to a group of medicines blocking the passage of impulses through particular nerves (known as anticholinergics),
• phenylephrine (as phenylephrine hydrochloride) which belongs to a group of medicines mimicking the effects of impulses conveyed through particular nerves (known as alpha sympathomimetics),
• lidocaine (as lidocaine hydrochloride) which belongs to a class of drugs called amide type local anaesthetics.
What it is used for
This medicine is used in adults only.
It will be administered by your ophthalmic surgeon by injection into the eye at the beginning of cataract surgery (cloudiness of the lens), in order to enlarge the pupil of your eye (mydriasis) and to obtain anaesthesia in your eye during the surgical procedure.
2. WHAT YOU NEED TO KNOW BEFORE YOU ARE GIVEN MYDRANE
You should not be given MYDRANE:
• if you are allergic to tropicamide, phenylephrine hydrochloride and/or lidocaine hydrochloride or to any of the other ingredients of this medicine (listed in section 6),
• if you are allergic to anaesthetics of the amide type,
• if you are allergic to atropine derivatives.
Warnings and precautions MYDRANE is not recommended:
• in combined cataract surgery with a certain type of eye surgery (vitrectomy),
• if the anterior part (anterior chamber) of your eye is shallow,
• if you have a history of acute increase of eye pressure (acute narrow angle glaucoma).
You should talk to your doctor in particular if you have:
• high blood pressure (hypertension),
• thickening of the arterial wall (atherosclerosis),
• any heart disease and particularly if it affects the heart rate,
• a contraindication to medicines that increased blood pressure (pressor amines) by general route,
• overactive thyroid gland (hyperthyroidism),
• prostate gland disorders,
• fits (epilepsy),
• any liver diseases or kidney problems,
• any problems with your breathing,
• loss of muscle function and weakness (myasthenia gravis).
Other medicines and MYDRANE
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Pregnancy, breast-feeding and fertility
This medicine should not be used:
• during pregnancy,
• during breast-feeding.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking any medicine. Driving and using machines
Mydrane has a moderate influence on the ability to drive and use machines. Consequently, you should not drive and/or use machines until vision is normal.
MYDRANE contains sodium
This medicinal product contains less than 1 mmol
sodium (23 mg) per dose, i.e. essentially “sodium-free".
3. HOW MYDRANE IS ADMINISTERED
You should only be given this medicine if you have demonstrated, at a previous visit, a satisfactory pupil dilation with topical mydriatic therapy.
Dose and method of administration
MYDRANE injection will be administered by an ophthalmic surgeon, under local anaesthesia, at the beginning of cataract surgery.
The recommended dose is 0.2 ml of solution, in only one injection. No additional dose should be injected as no additional effect has been shown and as increased loss of endothelial cells (cells of a layer covering the posterior surface of the cornea) has been observed. The same dose is used for both adults and the elderly. If you are given too much, or too little, MYDRANE:
Your medication will be given by an ophthalmic surgeon.
It is unlikely that you will be given an overdose.
If you have any further questions on the use of this medicine, ask your doctor, or pharmacist or nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most serious well known complications occurring during or after cataract surgery:
Uncommon: may affect up to 1 in 100 people
• Injury to the lens (posterior capsule rupture),
• Swelling of the retina (cystoid macular oedema).
Please seek urgent medical advice in this case.
Other side effects:
Uncommon: may affect up to 1 in 100 people
• Headache,
• Swelling of the cornea (keratitis), increased pressure in the eye, redness of the eye (ocular hyperaemia),
• High blood pressure (hypertension).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme.
Website: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE MYDRANE
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton, blister and ampoule. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
For single eye use only. This medicine should be used immediately after first opening of the ampoule.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What MYDRANE contains
• The active substances are tropicamide 0.04 mg, phenylephrine hydrochloride 0.62 mg and lidocaine hydrochloride 2 mg for each 0.2 ml dose, equivalent to 0.2 mg of tropicamide, 3.1 mg of phenylephrine hydrochloride and 10 mg of lidocaine hydrochloride for 1 ml.
• The other ingredients are: sodium chloride, disodium phosphate dodecahydrate, disodium phosphate dihydrate, disodium edetate and water for injections.
What MYDRANE looks like and contents of the pack MYDRANE is a clear, slightly brownish-yellow solution for injection, practically free from visible particles and supplied in a 1 ml brown glass ampoule. Each ampoule contains 0.6 ml of solution for injection and is presented in a sealed paper/PVC blister.
Each box contains 1 or 20 or 100 ampoules (with 5 micrometers filter needle(s) to be used only for the withdrawal of the vial contents). All components are for single-use only.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder LABORATOIRES THEA
12, RUE LOUIS BLERIOT
63017 CLERMONT-FERRAND CEDEX 2
FRANCE
Manufacturer
DELPHARM TOURS
RUE PAUL LANGEVIN
37170 CHAMBRAY LES TOURS
FRANCE
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Germany, Denmark, Greece, Finland, France, Croatia, Iceland, Italy, Luxembourg, The Netherlands, Poland, Portugal, Romania, Sweden, Slovenia, Slovak Republic,
This leaflet was last revised in 07/2015
If you would like any more information, or would like the leaflet in a different format, please contact Medical Information at THEA Pharmaceuticals Ltd, telephone number 0870 192 3283.
The following information is intended for medical or healthcare professionals only: Incompatibilities
No incompatibility with most commonly used products in cataract surgery was reported in literature with the active ingredients, and during clinical trials. For usual viscoelastics, this was also confirmed by pharmaceutical interaction test.
Warning
Do not use if the blister is damaged or broken. Open under aseptic conditions only. The content of the unopened blister is guaranteed to be sterile.
How to prepare and administer MYDRANE Single-eye use solution for intracameral use only. MYDRANE must be administered by intraocular injection into the anterior chamber of the eye (intracameral injection), by an ophthalmic surgeon, in the recommended aseptic conditions of cataract surgery. Before intracameral injection, the solution should be visually inspected and should only be used if it is clear, slightly brownish-yellow and practically free from visible particles.
The recommended dose is 0.2 ml of MYDRANE; no additional dose should be injected as no significant add-on effect has been demonstrated and as increased endothelial cell loss was observed.
The product should be used immediately after opening of the ampoule and not be reused for the other eye or any other patient.
To prepare MYDRANE for intracameral administration, please adhere to the following instructions:
1. Inspect unopened blister to ensure that it is intact. Peel open blister.
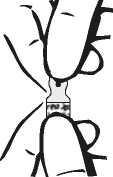
2. Break open the ampoule containing the drug product.
The One Point Cut (OPC) ampoule must be opened as follows: Hold the bottom part of the ampoule with the thumb pointing to the coloured point. Grasp the top of the ampoule with the other hand, positioning the thumb at the coloured point and press back to break at the existing cut under the point.

3. Assemble the 5-micron filter sterile needle (provided) onto a sterile syringe. Remove the 5-micron filter sterile needle protector and withdraw at least 0.2 ml of the solution for injection from the ampoule into the syringe.

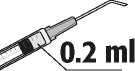
4
Disconnect the needle from the syringe and assemble the syringe with an appropriate anterior chamber cannula.
5. Carefully expel the air from the syringe. Adjust to 0.2 ml. The syringe is ready for injection.
6. Inject slowly the 0.2 ml syringe volume into the anterior chamber of the eye, in only one injection, through the side port or principal port.
After use, discard the remaining solution.
Do not keep it for subsequent use.
Any unused product or waste material should be disposed of in accordance with local requirements. Discard used needles in a sharps container.

N2380A20L03/0715 - 0000000