Nasacort 55 Mcg/Dose Nasal Spray Suspension
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
NASACORT 55 micrograms/dose, nasal spray, suspension
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Bottles of NASACORT contain either 6.5 g or 16.5 g of suspension (with 3.575 mg or 9.075 mg triamcinolone acetonide respectively). One delivered dose contains55 micrograms of triamcinolone acetonide.
Excipient with known effect: 15 micrograms of benzalkonium chloride/delivered dose.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Nasal spray, suspension.
It is an unscented, off-white, thixotropic suspension of microcrystalline triamcinolone acetonide in an aqueous medium.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
NASACORT is indicated for the treatment of symptoms of seasonal and
perennial allergic rhinitis.
4.2 Posology and method of administration
Posology
Patients aged 12 years and over: The recommended starting dose is 220 micrograms as 2 sprays in each nostril once daily. Once symptoms are controlled patients can be maintained on 110 micrograms (1 spray in each nostril once daily).
Paediatric patients aged 6 to 12 years: The recommended dose is 110 micrograms as 1 spray in each nostril once daily. In patients with more severe symptoms, a dose of 220 micrograms may be used. But once symptoms are controlled, patients should be maintained on the lowest effective dose. (see sections 4.4 and 5.1).
Special population Paediatric population
Until further evidence is available, continuous use beyond 3 months in children under 12 years is not recommended.
Method of administration
NASACORT is for nasal use only.
It is important to shake the bottle gently before each use.
Each actuation delivers 55 micrograms triamcinolone acetonide from the nose piece to the patient (estimated from in vitro testing) after an initial priming of 5 sprays until a fine mist is achieved. NASACORT will remain adequately primed for 2 weeks. If the product is unused for more than 2 weeks, then it can be adequately reprimed with one spray. The nozzle should be pointed away from you while you are doing this.
After using the spray: Wipe the nozzle carefully with a clean tissue or handkerchief, and replace the cap.
If the spray does not work and it may be blocked, clean it as follows. NEVER try to unblock it or enlarge the tiny spray hole with a pin or other sharp object because this will destroy the spray mechanism.
The nasal spray should be cleaned at least once a week or more often if it gets blocked.
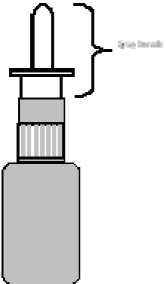
TO CLEAN THE SPRAY
1. Remove the cap and the spray nozzle only* (pull off).
2. Soak the cap and spray nozzle in warm water for a few minutes, and then rinse under cold running tap water.
3. Shake or tap off the excess water and allow to air-dry.
4. Re-fit the spray nozzle.
5. Prime the unit as necessary until a fine mist is produced and use as normal.
* Part as indicated on diagram below,
Also, the bottle should be discarded after 30 actuations or within one month of starting treatment (6.5 g pack), or after 120 actuations or within 2 months of starting treatment (16.5 g pack). Do not transfer any remaining suspension to another bottle.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
If there is any reason to suppose that adrenal function is impaired, care must be taken while transferring patients from systemic steroid treatment to NASACORT.
In clinical studies with NASACORT administered intranasally, the development of localised infections of the nose and pharynx with Candida albicans has rarely occurred. When such an infection develops it may require treatment with appropriate local therapy and temporary discontinuation of treatment with NASACORT.
Because of the inhibitory effect of corticosteroids on wound healing in patients who have experienced recent nasal septal ulcers, nasal surgery or trauma, NASACORT should be used with caution until healing has occurred.
Systemic effects of nasal corticosteroids may occur, particularly at high doses prescribed for prolonged periods. These effects are much less likely to occur than with oral corticosteroids and may vary in individual patients and between different corticosteroid preparations. Potential systemic effects may include Cushing’s syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, cataract, glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children).
Treatment with higher than recommended doses may result in clinically significant adrenal suppression. If there is evidence of using higher than recommended doses then additional systemic corticosteroid cover should be considered during periods of stress or elective surgery.
Glaucoma and/or cataracts have been reported in patients receiving nasal corticosteroids. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma and/or cataracts.
NASACORT contains benzalkonium chloride, an irritant, which may cause skin reactions.
Paediatric population
As experience with NASACORT in children under 6 years of age is limited, use in this age group is not recommended.
Reduction in growth velocity has been reported in children receiving nasal corticosteroids, including NASACORT at licensed doses. See section 5.1.
It is recommended that the height of children receiving treatment with nasal corticosteroids is regularly monitored. Therapy should be managed with the aim of reducing the dose of nasal corticosteroid, if possible, to the lowest dose at which effective control of symptoms is maintained. In addition, consideration should be given to referring the patient to a paediatric specialist. The long-term effects of reduction in growth velocity associated with nasal corticosteroids, including the impact on final adult height are unknown.
4.5 Interaction with other medicinal products and other forms of interaction
No interactions with other medicaments are known
4.6 Fertility, pregnancy and lactation
Clinical experience in pregnant women is limited. In animal studies, corticosteroids have been shown to induce teratogenic effects. Triamcinolone acetonide may pass into human breast milk. Triamcinolone acetonide should not be administered during pregnancy or lactation unless the therapeutic benefit to the mother is considered to outweigh the potential risk to the foetus/baby.
4.7 Effects on ability to drive and use machines
NASACORT has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
The adverse events reported in clinical trials with NASACORT most commonly involved the mucous membranes of the nose and throat.
The following terminologies have been used in order to classify the occurrence of adverse reactions:
Very common >1/10; Common >1/100 and <1/10; Uncommon > 1/1,000 and < 1/100; Rare > 1/10,000 and <1/1,000; Very rare < 1/10,000 and not known (cannot be estimated from the available data).
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness
The most frequent adverse reactions in adults and children 6 years of age and older were:
• Infections and infestations Common: flu syndrome, pharyngitis, rhinitis
• Immune system disorders
Not known: hypersensitivity (including rash, urticaria, pruritus and facial oedema)
• Psychiatric disorders Not known: insomnia
Nervous system disorders
Common: headache
Not known: dizziness, alterations of taste and smell
• Eye disorders
Not known: cataract, glaucoma, increased ocular pressure
• Respiratory, thoracic and mediastinal disorders Common: bronchitis, epistaxis, cough
Rare: nasal septum perforations
Not known: nasal irritation, dry mucous membrane, nasal congestion, sneezing, dyspnoea
• Gastrointestinal disorders Common: dyspepsia, tooth disorder Not known: nausea
• General disorders and administration site conditions Not known: fatigue
• Investigations
Not known: decreased blood cortisol
Reduction of growth velocity has been observed in children during a post-marketing clinical trial with NASACORT (see section 5.1)
Systemic effects of nasal corticosteroids may occur, particularly when prescribed at high doses for prolonged periods. Growth retardation has been reported in children receiving intranasal steroids.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
4.9 Overdose
Like any other nasally administered corticosteroid, acute overdosing with NASACORT is unlikely in view of the total amount of active ingredient present. In the event that the entire contents of the bottle were administered all at once, via either oral or nasal application, clinically significant systemic adverse events would most likely not result. The patient may experience some gastrointestinal upset if taken orally.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: DECONGESTANTS AND OTHER NASAL PREPARATIONS FOR TOPICAL USE, Corticosteroids, ATC code: R 01 AD11
Mechanism of action
Triamcinolone acetonide is a more potent derivative of triamcinolone and is approximately 8 times more potent than prednisone. Although the precise mechanism of corticosteroid antiallergic action is unknown, corticosteroids are very effective in the treatment of allergic diseases in man.
Pharmacodynamic effects
NASACORT does not have an immediate effect on allergic signs and symptoms. An improvement in some patient symptoms may be seen within the first day of treatment with NASACORT and relief may be expected in 3 to 4 days. When NASACORT is prematurely discontinued symptoms may not recur for several days.
In clinical studies performed in adults and children at doses up to 440 mcg/day intranasally, no suppression of the Hypothalamic-Pituitary-Adrenal (HPA) axis has been observed.
A one-year double-blind, placebo-controlled parallel group study in 298 treated pediatric patients (3 to 9 years of age) was conducted to assess the effect of NASACORT (once-daily dose of 110 micrograms) on growth velocity using stadiometry. From the primary analysis of evaluable patients (134 NASACORT and 133 placebo), the estimated growth velocity in the NASACORT group was 0.45 cm/year lower than that in the placebo group with 95% CI ranging between 0.11 to 0.78 cm/year lower than placebo. Difference between treatment groups started within 2 months of drug initiation. After stopping treatment during the 2-month follow-up period it was observed that the mean growth velocity in the treatment group returned to baseline (pre-treatment) values.
5.2 Pharmacokinetic properties
Single dose intranasal administration of 220 micrograms of NASACORT in normal adult subjects and in adult patients with allergic rhinitis demonstrated low absorption of triamcinolone acetonide. The mean peak plasma concentration was approximately 0.5ng/mL (range 0.1 to 1ng/mL) and occurred at 1.5 hours post dose. The mean plasma drug concentration was less than 0.06ng/mL at 12 hours and below the assay detection limit at 24 hours. The average terminal half life was 3.1 hours. Dose proportionality was demonstrated in normal subjects and in patients following a single intranasal dose of 110 micrograms or 220 micrograms NASACORT. Following multiple doses in paediatric patients, plasma drug concentrations, AUC, Cmax and Tmax were similar to those values observed in adult patients.
5.3 Preclinical safety data
In pre-clinical studies, only effects typical of glucocorticoids were observed.
Like other corticosteroids, triamcinolone acetonide (administered by inhalation or other routes) has been shown to be teratogenic in rats and rabbits, resulting in cleft palate and/or internal hydrocephaly and axial skeletal defects. Teratogenic effects, including CNS and cranial malformations, have also been observed in non-human primates.
No evidence of mutagenicity was detected in in-vitro gene mutation tests. Carcinogenicity assays in rodents show no increase in the incidence of individual tumour types.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
- microcrystalline cellulose and carmellose sodium (dispersible cellulose)
- polysorbate 80,
- purified water,
- anhydrous glucose,
- benzalkonium chloride (50% w/v solution)
- disodium edetate
- hydrochloric acid or sodium hydroxide (for pH-adjustment).
6.2 Incompatibilities
Not applicable
6.3 Shelf life
Unopened: 2 years
After first opening: 1 month for the 6.5g (30 actuation) pack and 2 months for the 16.5g (120 actuation) pack.
6.4 Special precautions for storage
Do not store above 25°C
For storage conditions after first opening of the medicinal product, see section 6.3.
6.5 Nature and contents of container
NASACORT is contained in a 20 mL high density polyethylene (HDPE) bottle fitted with a metered-dose spray pump unit.
One bottle of NASACORT contains either 6.5 g or 16.5 g of suspension, providing 30 or 120 actuations respectively.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
No special requirements
7
MARKETING AUTHORISATION HOLDER
Aventis Pharma Limited
One Onslow Street
Guildford
Surrey
GU1 4YS
United Kingdom and trading as:-
Sanofi-aventis or Sanofi
One Onslow Street
Guildford
Surrey
GU1 4YS
United Kingdom
8 MARKETING AUTHORISATION NUMBER
PL 04425/0287
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
28/02/1997 / 19/01/2007
10 DATE OF REVISION OF THE TEXT
29/06/2016