Nasacort 55 Micrograms/Dose Nasal Spray Suspension
Manufacturer and Licence Holder
This medicine is manufactured by Aventis Pharma, Holmes Chapel, UK, 72 London Road, Holmes Chapel, Crewe, Cheshire, CW4 8BE, United Kingdom and is procured from within the EU and repackaged by the Product Licence Holder: Lexon (UK) Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE.
If you have any questions or are not sure about anything, ask your doctor or pharmacist. They will have additional information about this medicine and will be able to advise you.
POM
PL 15184/1463 - Nasacort 55 micrograms, Nasal Spray Suspension
Nasacort is a registered trademark of Aventis Pharma S.A.
Leaflet revision date: 30/05/14
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
PATIENT INFORMATION LEAFLET Ref 1463/300514/1/f
Nasacort® 55 micrograms/dose, Nasal Spray Suspension
triamcinolone acetonide
5. HOW TO STORE NASACORT
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store above 25°C.
• Following the first opening Nasacort should be used within 2 months.
• Do not use Nasacort after the exipry date. This is marked on the carton and the bottle after EXP The exipry date refers to the last day of that month.
• If your doctor tells you to stop taking this medicine, return any unused medicine to your pharmacist (chemist) for safe disposal. Only keep this medicine, if your doctor tells you to.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Nasacort contains
• The active substance is triamcinolone acetonide. Each dose contains 55 micrograms of triamcinolone acetonide.
The other ingredients are microcrystalline cellulose and carmellose sodium (dispersible cellulose), polysorbate 80, purified water, anhydrous glucose, benzalkonium chloride (50% w/v solution), disodium edetate, hydrochloric acid or sodium hydroxide (for pH-adjustment).
What Nasacort looks like and the contents of the pack
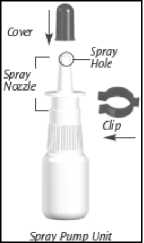
Nasacort is a suspension in a white plastic bottle which has a pump. The bottle has a protective blue cover to keep the nozzle clean and a blue plastic clip to stop it from spraying accidentally.
Each bottle contains 120 doses.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See Section 4
Your medicine is called Nasacort 55 micrograms/dose, Nasal Spray Suspension but will be referred to as Nasacort throughout the rest of the leaflet
What is in this leaflet
1. What Nasacort is and what it is used for
2. What you need to know before you use Nasacort
3. How to use Nasacort
4. Possible side effects
5. How to store Nasacort
6. Contents of the pack and other information
1. WHAT NASACORT IS AND WHAT IT IS USED FOR
Nasacort contains a medicine called triamcinolone acetonide. This belongs to a group of medicines called corticosteroids which means it is a type of steroid. It is given as a spray in the nose to treat the nasal symptoms of allergic rhinitis.
Nasal symptoms of allergy include sneezing, itching, and having a blocked, stuffy or runny nose. These can be caused by things such as:
• Animal fur or house dust mites. This type of allergy can happen at any time of the year and is called ‘perennial allergic rhinitis'.
• Pollen. This type of allergy, such as hay fever, can be caused by different pollens in different seasons of the year. This is called ‘seasonal allergic rhinitis'.
This medicine only works if used on a regular basis and may not help your symptoms straight away. It helps some people within the first day of treatment, however, for other people it may take 3 to 4 days to feel a relief.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE NASACORT Do not use Nasacort:
• If you are allergic to triamcinolone acetonide or any of the other ingredients of this medicine (listed in section 6)
Signs of an allergic reaction to Nasacort include: a rash (hives), itching, swallowing or breathing problems, swelling of your lips, face, throat or tongue.
Warning and precautions
Talk to your doctor or pharmacist before using
Nasacort:
• If you have any infection of the nose or throat that is not treated. If you get a fungal infection while using Nasacort, stop using the spray until the infection has been treated.
• If you have recently had a nose operation, or had an injury or ulcer in the nose.
• If you are being transferred from steroid injections or tablets to Nasacort spray.
• If you have had glaucoma or cataracts.
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using this medicine.
Children (under 6 years)
This medicine is not recommended for use in children under 6 years of age.
Operations or times of stress
Your doctor may advise you to take a higher than normal dose of this medicine for medical reasons. If your dose is increased, tell your doctor if you are going to have an operation or are feeling unwell. This is because higher than normal doses of this medicine can lower your body's ability to heal or cope with stress. If this happens, your doctor may decide you need further treatment with another medicine to help.


Other medicines and Nasacort
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This is because Nasacort can affect the way some other medicines work. Also some medicines can affect the way Nasacort works.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Nasacort has no or negligible influence on the ability to drive or use machines.
Nasacort contains benzalkonium chloride
which can be an irritant and may cause skin reactions
3. HOW TO USE NASACORT
NASACORT is for nasal use only.
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The medicine only works if it is used regularly. It can take 3 to 4 days before you notice your symptoms getting better.
How much Nasacort to use Adults and children (over 12 years)
• The usual starting dose is 2 sprays in each nostril each day
• Once the symptoms of allergy are under control, the dose may be lowered to 1 spray in each nostril each day
Children (6 to 12 years)
• The usual dose is 1 spray in each nostril each day
• If the symptoms do not go away, then the dose may be increased to 2 sprays in each nostril each day
• The dose can then be lowered again to 1 spray in each nostril each day
• Do not use Nasacort for more than 3 months in children under 12 years old
How to use the spray
Before using your nasal spray, blow your nose
gently to clear your nostrils.
1. Preparing the bottle
• Remove the blue cover by pulling upwards
• Pull off the blue plastic clip
• Shake the bottle gently before use
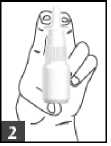
2. If you are using the spray for the first time
• Hold the bottle upright
• Point the spray away from you while doing this
• Fill the pump with spray by pressing the nozzle downwards.
This is called priming
• Press and release it 5 times
• Do this until a fine spray is produced
• The spray is now ready to use
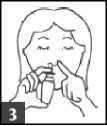
3. Using the spray
• Close one nostril with your finger
• Hold the bottle upright and put the nozzle into the other nostril as far as is comfortable
• Breathe in gently through your nose with your mouth closed
• While you are doing this, press the nozzle to deliver one spray
4. Then breath out through your mouth
5. Repeat steps 3 and 4 if you have to spray again in the same nostril and for the other nostril
6. After using the spray
• To keep the spray nozzle clean, wipe it carefully with a clean tissue or handkerchief after each use
• Press the blue plastic clip back into place to stop accidental release of the spray
• Replace the blue cover over the nozzle
If the nasal spray has not been used for more
than 2 weeks:
• It needs to be primed again, to fill the nozzle with the spray
• The nozzle should be pointed away from you while you are doing this
• To prime, spray into the air once before use
• Always shake the bottle gently before use
Cleaning the spray
If the spray does not work, the nozzle may be blocked. Never try to unblock it or enlarge the tiny spray hole with a pin or other sharp objects. This is because it can stop the spray from working.
The nasal spray should be cleaned at least once a week. It can be cleaned more often if it gets blocked.
Instructions for cleaning the spray:
1. Remove the blue cover
2. Gently pull off the spray nozzle only
3. Soak the blue cover and spray nozzle in warm water for a few minutes
4. Rinse under the cold running tap water
5. Shake or tap to remove any water that is left
6. Allow to dry in the air
7. Re-fit the spray nozzle
8. Prime the nasal spray until a fine mist is formed
9. Use as normal
If you use more Nasacort than you should
It is important that you take your dose as stated on the pharmacist's label or as advised by your doctor. You should use only as much as your doctor recommends; using more or less may make your symptoms worse.
An overdose is unlikely to cause problems, however, if you have taken the entire contents of the bottle by mouth you may have stomach or gut discomfort. Talk to a doctor if you use more Nasacort than you should.
If you forget to use Nasacort
If you have forgotten to use Nasacort, use it as soon as you remember. Do not use a double dose to make up for a forgotten dose.
If you stop using Nasacort
If you stop using this medicine, your symptoms may return within a few days.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Nasacort and see a doctor or go to a hospital straight away if:
• you have an allergic reaction to Nasacort. The signs (frequency not known) may include: a rash (hives), itching, swallowing or breathing problems, swelling of your lips, face, throat or tongue.
Common side effects (may affect up to 1 in 10
people)
• Runny nose, headache, sore throat and/or cough
• Nosebleeds
• Inflammation/irritation of the airways (bronchitis)
• Heartburn or indigestion
• Flu-like symptoms (fever, muscle pain, weakness and/or fatigue)
• Problems with teeth
Other side effects (Not known: frequency cannot be estimated from the available data)
• Irritation and dryness on the inside of your nose
• Sinuses become congested or blocked
• Sneezing
• Changes in the way things taste or smell
• Feeling sick (nausea)
• Sleeping problems, feeling dizzy or tired
• Shortness of breath (dyspnoea)
• A decrease in the levels of cortisol in the blood (lab value)
• Cloudiness of the lens in the eye (cataract), elevated pressure inside the eyeball (glaucoma)
In some people, Nasacort can cause damage to the middle part of the inside of the nose (called ‘nasal septum'). Discuss any worries you may have about this with your doctor or pharmacist.
Additional side effects in Children
If your child has been using this medicine, it can affect how fast your child grows. This means that your doctor will need to regularly check your child's height and therefore your doctor may lower the dose. In addition, your doctor may consider referring your child to a paediatric specialist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
Ref: 1463/300514/1/B