Nasofan Aqueous 50 Microgram Nasal Spray
TEVA UK Ref: 231-30-87161-H LEA FLUTICASONE (NASOFAN) 50MCG SPRAY 150 IVAX <OPA II Version: 2 10 May 2016
mu
EXAMPLE
mu

Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
See Section 4.
|
Dimensions: Length: 297 mm Width: 148 mm |
Colours Used: ^^antone^s7^^ | |||
|
UK |
Origination |
I Template |
1. What Nasofan Nasal Spray is and what it is used for
2. What you need to know before you use Nasofan Nasal Spray
3. How to use Nasofan Nasal Spray
4. Possible side effects
5. How to store Nasofan Nasal Spray
6. Contents of the pack and other information
Your medicine is called Nasofan Aqueous 50 microgram Nasal Spray (described as 'Nasofan Nasal Spray' throughout this leaflet) and contains 50 micrograms of the active ingredient, fluticasone propionate, in each spray. Fluticasone propionate is one of a group of medicines known as corticosteroids. Nasofan Nasal Spray has anti-inflammatory properties. When sprayed into your nose it will reduce swelling and irritation. It is used to prevent and treat seasonal allergic rhinitis (e.g. hayfever) and perennial rhinitis (e.g. blocked or runny nose and sneezing and itching caused by house dust mites or animals such as cats and dogs). It can be used by adults and children aged 4 years and older.
2. What you need to know before you use Nasofan Nasal Spray
Do not use Nasofan Nasal Spray :
• if you are allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Nasofan Nasal
• if you have ever had an operation to your nose.
• if you are suffering or have recently suffered from an infection of the nasal airways.
• if you are suffering or have recently suffered from any type of untreated infection or from tuberculosis or ocular herpes.
• if you have recently been treated with injected steroids, or you have been taking oral steroids for a long time.
Nasofan Nasal Spray will usually control seasonally allergy rhinitis (hay fever) however if you are exposed to excessive
controlling other symptoms such as itching eyes. Please consult your doctor in such an event.
Other medicines and Nasofan Nasal Spray
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Some medicines can interfere with Nasofan Nasal Spray, in particular tell your doctor or pharmacist if you are taking:
• Any medicines used in the treatment of fungal infections (e.g. ketoconazole)
• Any antiviral medicine such as those used to treat HIV infection (e.g. ritonavir)
Pregnancy and breast-feeding If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
The medicinal product has no or negligible influence on the ability to drive and use machines.
Nasofan Nasal Spray contains benzalkonium chloride solution
Nasofan Nasal Spray contains the ingredient benzalkonium chloride solution (40 micrograms per delivered dose) which is an irritant and may cause skin reactions. If used for longer periods, the preservative benzalkonium chloride may cause nasal mucosa swelling. In case of such a reaction (persistently congested nose) medicinal products for nasal use without preservative should be used if possible. It may also cause bronchospasm. Unless such products are available another pharmaceutical form should be taken. It may also cause bronchospasm.
3. How to use Nasofan Nasal Spray
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are
The recommended dose is as follows:
Adults (including the elderly) and children aged 12 years and older:
When you first start using Nasofan Nasal Spray you normally take two sprays in each nostril once a day, preferably in the morning. Your doctor may increase this to a maximum of two sprays into each nostril twice a day.
Once your symptoms are under control your doctor may reduce your dose to one spray into each nostril once a day. If the use of such a reduced dose signals a worsening in your symptoms, your dose may be increased back to the starting dose. Children aged 4 to 11 yearsa:
For children aged between 4-11 years old the dose is normally one spray in each nostril once a day, preferably in the morning. Your doctor may increase this to a maximum of one spray into each nostril twice a day.
This product is not suitable for children under 4 years old.
Your doctor will prescribe the lowest dose of Nasofan Nasal Spray capable of effectively controlling your symptoms.
It may take a few days for this medicine to work. Do not stop using your medicine unless you are told to by a doctor.
You must not take a larger dose, or use your Nasofan Nasal Spray more often, than is prescribed by your doctor. It is important not to use more of your medicine than your doctor has told you to.
If your eyes remain itchy or watery as a result of hayfever despite using this medicine tell your doctor. He/she may be able to give you another medicine to treat your eye symptoms.
Before you use your nasal spray
Your Nasofan Nasal Spray has a dust cap that protects the nozzle and keeps it clean - this must be taken off prior to using the spray and then replaced after use.
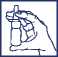
When your Nasofan Nasal Spray is new the bottle must be primed as follows:-
1. Shake the bottle gently and then remove the dust cap.
2. Hold the bottle upright with your thumb under the bottle and your index finger and middle finger on either side of the nozzle. Ensure that the nozzle is pointing away from you when you do this.
3. Press down with fingers in order to pump the spray
4. Repeat steps 2 and 3 until a further five times - the bottle is now primed.
If you have not used your Nasofan Nasal Spray for 7 days prime until a fine mist is produced.
If after attempting to prime the bottle the spray still
does not work and you think it may be blocked you
may clean the spray using the following procedure:-
Cleaning your nasal spray
1. Remove the dust cap.
2. Pull the white collar upwards to remove the nozzle.
3. Place the nozzle and dust cap in warm water and soak for a few minutes, and then rinse by placing under a running tap.
4. Shake off the excess water and allow the nozzle and dust cap to dry in a warm (not hot) place.
5. Re-fit the nozzle.
6. 'Prime' the bottle as necessary by pumping the spray a few times until a fine mist is produced.
• You should clean your nasal spray at least once a week to stop it from getting blocked up. Additional cleaning is required when your spray becomes blocked.
• You must NEVER attempt to unblock or enlarge the spray hole with a pin or other sharp object because this will destroy the spray mechanism.
Using your nasal spray
1. Shake the bottle and remove the dust cap.
2. Gently blow your nose.
3. Close one nostril by pressing your finger against it and place the nozzle of the spray in your other nostril. Tilt your head forward slightly so that the bottle is kept upright.
4. Breathe in slowly through your open nostril and, at the same time, press the collar of the nozzle down firmly with your fingers to squirt a spray of fine mist into your nostril.
5. Breathe out through your mouth. Repeat step 4 to take a second spray into the same
6. Remove the nozzle from this nostril and breathe out through your mouth.
7. Repeat steps 3 to 6 with your other



|
Dimensions: Length: 297 mm Width: 148 mm |
Colours Used: ^^antoneSS^^ | |||
|
UK |
Artwork Origination |
1 Template |
mu
EXAMPLE
mu
After using your nasal spray
• Wipe the nozzle carefully with a clean tissue and put the dust cap back on.
If you use more Nasofan Nasal Spray than you should
It is important that you take your dose as stated on the pharmacist's label or as advised by your doctor. You should use only as much as your doctor recommends; using more or less may make your symptoms worse.
If you accidentally use more Nasofan Nasal Spray than you were told to use please inform your doctor.
Take this leaflet, and your Nasofan Nasal Spray, to show the
If you forget to use Nasofan Nasal Spray
Do not take a double dose to make up for a forgotten dose. If you forget to take a dose at the right time, take it as soon as you remember. If it is almost time to take the next dose, wait until then and then carry on as before.
If you stop using Nasofan Nasal Spray Your nose symptoms may only start to improve after you have been using your medicine for a few days - therefore it is very important that you use your medicine regularly as prescribed, and that you keep using your medicine unless your doctor tells you to stop, even if you feel better.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you are using high doses of Nasofan Nasal Spray you may require extra steroids in times of extreme stress, or during admission to hospital after a serious accident or injury, or before a surgical operation. Treatment with nasal corticosteroids may affect the production of steroids in the body. The likelihood of such an incidence is increased by use of a high dose over a long period of time. Your doctor will help prevent this happening by prescribing the lowest dose of steroid capable of adequately controlling your symptoms.
Some side effects are more serious than others and if you should experience any of the following events you should discontinue taking Nasofan Nasal Spray and consult with your doctor as soon as possible:-
Side effects occurring very commonly (probably affecting more than 1 in 10patients taking the Nasofan Nasal Spray)
• epistaxis (nose bleeds).
Side effects occurring commonly (probably affecting up to 1 in 10 patients taking the Nasofan Nasal Spray)
• headache.
• unpleasant taste in the mouth or an unpleasant smell in
• dryness and irritation of the throat and nasal passages and sneezing.
Side effects occurring rarely (probably affecting fewer than 1 in every 1,000 patients taking Nasofan Nasal Spray)
• severe allergic reaction giving rise to the sudden onset of a rash, swelling (usually of the tongue, face or lips) or difficulty in breathing.
• bronchospasm (the narrowing of the airways in the lungs). Side effects occurring very rarely (probably affecting fewer than 1 in every 10,000 patients taking the Nasofan Nasal Spray)
• glaucoma (raised pressure in the eye) and cataracts (clouding of the lens in the eye) have been reported following prolonged treatment.
• perforation of the nasal septum (the dividing partition in the nose) and ulceration to the nose's mucus membranes -although these usually impact on patients who have had previous surgery to the nose.
Additional side effects in children and adolescents Children may grow more slowly than others, and therefore children receiving treatment with nasal corticosteroids over a long period of time will have their height checked regularly by their doctor. Your doctor will help prevent this happening by prescribing the lowest dose of steroid capable of adequately controlling the symptoms.
Reporting of side effects
If you get side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www mhra gov uk/yellowcard
By reporting side effects you can help provide m information on the safety of this medicine.
This medicinal product is authorised in the Member States of the EEA under the following names
|
5. How to store Nasofan Nasal Spray Keep this medicine out of the sight and reach of children. |
Czech Republic Germany |
Nasofan, nasni sprej Flutica-TEVA 50 Mikrogramm Nasenspray, Suspension |
|
Do not use this medicine after the expiry date which is stated on the carton and bottle after EXP. The expiry date refers to the last day of that month. |
Denmark |
Fluticasonpropionat "Teva" 50 mikrogram/dosis, nasespray, |
|
Do not store above 25°C. Do not throw away any medicines via wastewater or |
Sp™ |
Fluticasona Teva 50 microgram i suspension para pulverizacion |
|
household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect |
Finland |
Nasofan 50 mikrog/annos |
|
the environment. |
Hungary |
Flutirin orrspray |
Iceland
This leaflet w last revised in April 2016
-O
6. Contents of the pack and other information
What Nasofan Nasal Spray contains
The active substance is fluticasone propionate. Each metered spray contains 50 micrograms of fluticasone propionate.
The other ingredients are glucose anhydrous, dispersible cellulose, phenylethyl alcohol, benzalkonium chloride solution (40 micrograms per delivered dose), polysorbate 80, and purified water.
What Nasofan Nasal Spray looks like and contents of the
Nasofan Nasal Spray consists of a white, opaque suspension contained within an amber glass multi-dose bottle fitted with an atomising metering pump to create the spray. Each bottle of Nasofan Nasal Spray contains suspension capable of delivering 60, 120, 150, 240 (2 bottles each containing 120 sprays) or 360 (3 bottles each containing 120 sprays) sprays. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder: IVAX Pharmaceuticals UK, WF10 5HX
Manufacturer: TEVA Czech Industries s.r.o, Opava, Czech Republic.
Ireland
Italy
The Netherlands Poland
Portugal
Slovak Republic United Kingdom
Nasofan 50 mikrog/skammt nefuSi, dreifa
Nasofan Aqueous 50 microgram Nasal Spray Suspension Nasofan 50 microgrammi Spray Nasale
Fluticasonpropionaat 50 A, neusspray 50 microgram/dosis Fanipos, 50 mikrogramow/dawk^ donosow^ aerosol donosa,
Fluticasona Nasofan 50
microgramas Suspensao para
pulverizapao nasal
Nasofan 50 mikrogramova nosova
aerodisperzia aer nas
Nasofan Aqueous 50 microgram
Nasal Spray
