Night Nurse
July 2011
1.3.2 Mock-up leaflet
Night Nurse Hot Lemon Menthol Powder for Oral Solution
PL 00079/0671
_GlaxoSmithKline Consumer Healthcare, UK.
1.3.2 Mock-up
Leaflet
A mock-up of the leaflet proposed for marketing Night Nurse Hot Lemon Menthol Powder for Oral Solution is provided on the following page.
150mm
40mm Lead in to First bar
THIS CODE READ 1 IN THIS DIRECTION
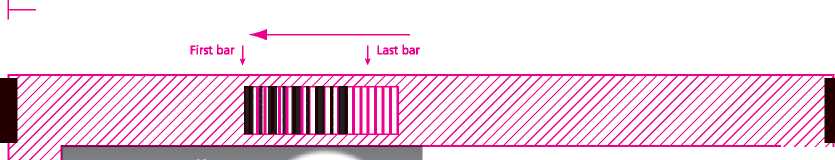
|—| 3mm
12mm

Hot Lemon Menthol
Powder for Oral Solution
Paracetamol, Dextromethorphan, Promethazine
lease read right through this leaflet before you start ising this medicine. This medicine is available ithout prescription, but you still need to use this edicine carefully to get the best results from it.
Keep this leaflet you may need to read it again.
If you have any further questions, ask your pharmacist.
n this leaflet:
1. What does this medicine do :. Check before you take this medicine 3. How to take this medicine Possible side effects How to store this medicine Further information
1. What does this medicine do
I ight Nurse Hot Lemon Menthol Powder for Oral iolution is used for the night-time relief of the major ymptoms of colds, chills and flu. The medicine contains three active ingredients. Paracetamol is a painkiller and educes your temperature when you have a fever, romethazine hydrochloride is an antihistamine which dries up a runny nose and aids restful sleep, extromethorphan hydrobromide is a cough uppressant that helps relieve dry or tickly coughs. This edicine also helps to relieve a sore throat.
. Check before you take this medicine
Do not take this medicine:
dextromethorphan hydrobromide or to any of the other ingredients (listed in Section 6).
• if you have a chest infection, worsening asthma or severe respiratory problems.
• if you are taking or have taken monoamine oxidase inhibitors (MAOIs) prescribed for depression in the last two weeks.
• if you are having a pregnancy test carried out on your urine.
Do not take with any other paracetamol-containing products.
Do not take with any other cold, flu or decongestant products or antihistamines.
H Take special care with this medicine:
• Do not drive or operate machinery. The medicine may cause drowsiness.
• Do not drink alcohol while using this medicine.
• If your symptoms do not improve, or if you develop a high temperature, skin rash or persistent headache, see a doctor.
D Ask your doctor before you take this medicine:
• if you have heart, kidney or liver problems.
• if you have glaucoma, epilepsy, difficulty passing urine or prostate problems.
• if you have long term or persistent cough (such as with asthma and emphysema), or cough with excessive phlegm.
• if you are on a controlled-sodium diet. Each sachet contains 122 mg of sodium.
• if you have phenylketonuria. This medicine contains aspartame, a source of phenylalanine which may be harmful to you if you suffer from phenylketonuria.
H If you are taking other medicines
Please talk to your doctor or pharmacist before taking this medicine if you are taking any prescribed medicines; particularly metodopramide or domperldone (for nausea or vomiting); colestyramine (to lower blood cholesterol); medicines which give you blurred vision, a dry mouth or make you drowsy; blood thinning drugs
-e-
if you have ever had an allergic reaction to
paracetamol, promethazine hydrochloride,

12mm
9mm
|—| 3mm
12mm
150mm

(anticoagulants e.g. warfarin); medicines for anxiety ' or depression (e.g. selective serotonin reuptake / inhibitor (SSRI) or tricyclic antidepressant) or to help ' you sleep.
How to take this medicine
Empty contents of one sachet into a mug. Half fill with very hot water. Stir well. Add cold water as necessary.
Adults and children aged 16 years and above: One sachet to be taken just before going to bed.
• Do not take this medicine if you have already taken 4 doses of a paracetamol-containing product during the day.
• Only take one dose of this medicine per night.
• Do not take more than the recommended dose.
• Do not take for more than 3 days.
• Do not give to children under 16 years.
If you take too much
Immediate medical advice should be sought in the event of an overdose, even if you feel well, because of the risk of delayed, serious liver damage.
If your symptoms persist, see your doctor.
. Possible side effects
Like all medicines this medicine can have side effects, but not everyone gets them. Older people are more prone to these side effects.
he following side effects may occur:
Drowsiness, dizziness, blurred vision, difficulty oncentrating, unsteadiness, clumsiness, headache, dry mouth.
Stop taking this medicine and tell your doctor immediately if you experience:
Allergic reactions which may be severe such as skin rash, itching sometimes with swelling of the mouth or face or shortness of breath Skin rash or peeling or mouth ulcers
• Breathing problems. These are more likely if you have experienced them before when taking other painkillers (such as ibuprofen and aspirin)
• Unexplained bruising and bleeding
• Confusion, feeling restless, sweating, shaking, shivering, sudden jerks of muscles or increased blood pressure
• Difficulty passing urine
• Nausea, sudden weight loss, loss of appetite and yellowing of the eyes and skin.
These effects are likely to be rare.
If you do get any side effects, even those not mentioned in this leaflet, tell your doctor or pharmacist.
5. How to store this medicine
Keep out of the reach and sight of children.
Do not take this medicine after the 'EXP' date shown on the pack.
Do not store above 25°C.
6. Further information
Each sachet of powder for oral solution contains the active ingredients: Paracetamol 1000 mg. Promethazine Hydrochloride 20 mg and Dextromethorphan Hydrobromide 15 mg.
Also includes: maltodextrin, sucralose, citric acid, tartaric acid, sodium citrate, acesulfame potassium (E950), aspartame (E951), quinoline yellow (E104), powdered menthol flavour, and lemon flavour.
This product is available in packs of 5 sachets.
The marketing authorisation holder is
GlaxoSmithKline Consumer Healthcare, Brentford, TW8 9GS, U.K. and all enquiries should be sent to this address.
The manufacturer is Wrafton Laboratories Ltd, Wrafton, Braunton, North Devon, EX33 2DL, U.K.
This leaflet was prepared in July 2011.
Night-Nurse is a registered trade mark of the GlaxoSmithKline group of companies.
-0
9 GlaxoSmithKline
6107835 L610019/01

12mm
Last
bar j
► First bar
9mm
-0
THIS CODE READ IN THIS DIRECTION
40mm Lead in to First bar