Nimbex Forte 5Mg/Ml Solution For Injection/Infusion
v3

GlaxoSmithKline
10000000122937
Information for the Physician
Nimbex®
cisatracurium
Please refer to the Summary of Product Characteristics (SPC) for further details on this product.
Trade name of the medicinal product
Nimbex 2 mg/ml solution for injection/infusion Nimbex Forte 5 mg/ml solution for injection/infusion
Qualitative and quantitative composition
Nimbex 2 mg/ml, solution for injection:
Cisatracurium 2 mg as cisatracurium besilate 2.68 mg per 1 ml one ampoule of 10 ml contains 20 mg of cisatracurium one ampoule of 25 ml contains 50 mg of cisatracurium Nimbex Forte 5 mg/ml, solution for injection:
Cisatracurium 5 mg as cisatracurium besilate 6.70 mg per 1 ml
one vial of 30 ml contains 150 mg of cisatracurium
Pharmaceutical form
Solution for injection/infusion.
Colourless to pale yellow or greenish yellow solution. Practically free from visible particulate matter.
Therapeutic indications
Nimbex is indicated for use during surgical and other procedures in adults and children aged 1 month and over. Nimbex is also indicated for use in adults requiring intensive care. Nimbex can be used as an adjunct to general anaesthesia, or sedation in the Intensive Care Unit (ICU) to relax skeletal muscles, and to facilitate tracheal intubation and mechanical ventilation.
Posology and method of administration
Nimbex should be only administered by or under the supervision of anaesthetists or other clinicians who are familiar with the use and action of neuromuscular blocking agents. Facilities for tracheal intubation, and maintenance of pulmonary ventilation and adequate arterial oxygenation have to be available.
Please note that Nimbex should not be mixed in the same syringe or administered simultaneously through the same needle as propofol injectable emulsion or with alkaline solutions such as sodium thiopentone.
Nimbex contains no antimicrobial preservative and is intended for single patient use.
Monitoring advice
As with other neuromuscular blocking agents, monitoring of neuromuscular function is recommended during the use of Nimbex in order to individualise dosage requirements.
Use by intravenous bolus injection Dosage in adults
Tracheal Intubation. The recommended intubation dose of Nimbex for adults is 0.15 mg/kg (body weight). This dose produced good to excellent conditions for tracheal intubation 120 seconds after administration of Nimbex, following induction of anaesthesia with propofol.
Higher doses will shorten the time to onset of neuromuscular block.
Table 1 summarises mean pharmacodynamic data when Nimbex was administered at doses of 0.1 to 0.4 mg/kg (body weight) to healthy adult patients during opioid (thiopentone/fentanyl/midazolam) or propofol anaesthesia.
Table 1: Mean Pharmacodynamic Data Following a Range of Cisatracurium Doses
|
Initial NIMBEX Dose mg/kg (body weight) |
Anaesthetic Background |
Time to 90% T11 Suppression (minutes) |
Time to Maximum T11 Suppression (minutes) |
Time to 25% Spontaneous T11Recovery (minutes) |
|
0.1 |
Opioid |
3.4 |
4.8 |
45 |
|
0.15 |
Propofol |
2.6 |
3.5 |
55 |
|
0.2 |
Opioid |
2.4 |
2.9 |
65 |
|
0.4 |
Opioid |
1.5 |
1.9 |
91 |
Dosage in paediatric patients
Tracheal Intubation (paediatric patients aged 1 month to 12 years):
As in adults, the recommended intubation dose of Nimbex is 0.15 mg/kg (body weight) administered rapidly over 5 to 10 seconds. This dose produces good to excellent conditions for tracheal intubation 120 seconds following injection of Nimbex. Pharmacodynamic data for this dose are presented in the tables 2, 3 and 4..
Nimbex has not been studied for intubation in ASA Class III-IV paediatric patients. There are limited data on the use of Nimbex in paediatric patients under 2 years of age undergoing prolonged or major surgery.
In paediatric patients aged 1 month to 12 years, Nimbex has a shorter clinically effective duration and a faster spontaneous recovery profile than those observed in adults under similar anaesthetic conditions. Small differences in the pharmacodynamic profile were observed between the age ranges 1 to 11 months and 1 to 12 years which are summarised in the tables 2 and 3.
Table 2: Paediatric Patients aged 1 to 11 months
|
NIMBEX Dose mg/kg (body weight) |
Anaesthetic Background |
Time to 90% Suppression (minutes) |
Time to Maximum Suppression (minutes) |
Time to 25% Spontaneous T1 Recovery (minutes) |
|
0.15 |
Halothane |
1.4 |
2.0 |
52 |
|
0.15 |
Opioid |
1.4 |
1.9 |
47 |
|
Table 3: Paediatric Patients aged 1 to 12 years | ||||
|
NIMBEX Dose mg/kg (body weight) |
Anaesthetic Background |
Time to 90% Suppression (minutes) |
Time to Maximum Suppression (minutes) |
Time to 25% Spontaneous T1 Recovery (minutes) |
|
0.15 |
Halothane |
2.3 |
3.0 |
43 |
|
0.15 |
Opioid |
2.6 |
3.6 |
38 |
When Nimbex is not required for intubation: A dose of less than 0.15 mg/kg can be used. Pharmacodynamic data for doses of 0.08 and 0.1 mg/kg for paediatric patients aged 2 to 12 years are presented in the table below:
Table 4: Paediatric patients aged 2 to 12 years
|
NIMBEX Dose mg/kg (body weight) |
Anaesthetic Background |
Time to 90% Suppression (minutes) |
Time to Maximum Suppression (minutes) |
Time to 25% Spontaneous T1 Recovery (minutes) |
|
0.08 |
Halothane |
1.7 |
2.5 |
31 |
|
0.1 |
Opioid |
1.7 |
2.8 |
28 |
Administration of Nimbex following suxamethonium has not been studied in paediatric patients.
Halothane may be expected to extend the clinically effective duration of a dose of Nimbex by up to 20%. No information is available on the use of Nimbex in children during anaesthesia with other halogenated fluorocarbon anaesthetic agents, but these agents may also be expected to extend the clinically effective duration of a dose of Nimbex.
Maintenance (paediatric patients aged 2-12 years). Neuromuscular block can be extended with maintenance doses of Nimbex. In paediatric patients aged 2 to 12 years, a dose of 0.02 mg/kg (body weight) provides approximately 9 minutes of additional clinically effective neuromuscular block during halothane anaesthesia. Consecutive maintenance doses do not result in progressive prolongation of effect.
There are insufficient data to make a specific recommendation for maintenance dosing in paediatric patients under 2 years of age. However, very limited data from clinical studies in paediatric patients under 2 years of age suggest that a maintenance dose of 0.03 mg/kg may extend clinically effective neuromuscular block for a period of up to 25 minutes during opioid anaesthesia.
Spontaneous Recovery. Once recovery from neuromuscular block is underway, the rate is independent of the Nimbex dose administered. During opioid or halothane anaesthesia, the median times from 25 to 75% and from 5 to 95% recovery are approximately 11 and 28 minutes, respectively. Reversal. Neuromuscular block following Nimbex administration is readily reversible with standard doses of anti-cholinesterase agents. The mean times from 25 to 75% recovery and to full clinical recovery (T4:T1 ratio > 0.7) are approximately 2 and 5 minutes respectively, following administration of the reversal agent at an average of 13% T1 recovery.
Use by intravenous infusion
Dosage in adults and children aged 2 to 12 years
Maintenance of neuromuscular block may be achieved by infusion of Nimbex. An initial infusion rate of 3 gg/kg (body weight)/min (0.18 mg/kg/hr) is recommended to restore 89 to 99% T1 suppression following evidence of spontaneous recovery. After an initial period of stabilisation of neuromuscular block, a rate of 1 to 2 gg/kg (body weight)/min (0.06 to 0.12 mg/kg/hr) should be adequate to maintain block in this range in most patients.
Reduction of the infusion rate by up to 40% may be required when Nimbex is administered during isoflurane or enflurane anaesthesia.
The infusion rate will depend upon the concentration of cisatracurium in the infusion solution, the desired degree of neuromuscular block, and the patient's weight. Table 5 provides guidelines for delivery of undiluted Nimbex.
Table 5: Infusion Delivery Rate of Nimbex injection 2 mg/ml
|
Patient (body weight) (kg) |
Dose (gg/kg/min) |
Infusion Rate | |||
|
1.0 |
1.5 |
2.0 |
3.0 | ||
|
20 |
0.6 |
0.9 |
1.2 |
1.8 |
ml/hr |
|
70 |
2.1 |
3.2 |
4.2 |
6.3 |
ml/hr |
|
100 |
3.0 |
4.5 |
6.0 |
9.0 |
ml/hr |
Steady rate continuous infusion of Nimbex is not associated with a progressive increase or decrease in neuromuscular blocking effect.

GlaxoSmithKline
2 What you need to know before you are given Nimbex
INI II I
Package Leaflet: Information for the User
Nimbex®
cisatracurium
Nimbex 2 mg/ml solution for injection/ infusion
Nimbex Forte 5 mg/ml solution for injection/ infusion
Read all of this leaflet carefully before
you are given this medicine because it
contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor.
• If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1 What Nimbex is and what it is used for
2 What you need to know before you are given Nimbex
3 How Nimbex is given
4 Possible side effects
5 How to store Nimbex
6 Contents of the pack and other information
1 What Nimbex is and what it is used for
Nimbex contains a medicine called cisatracurium. This belongs to a group of medicines called muscle relaxants.
Nimbex is used:
• to relax muscles during operations on adults and children over 1 month of age, including heart surgery
• to help insert a tube into the windpipe (tracheal intubation), if a person needs help to breathe
• to relax the muscles of adults in intensive care. Ask your doctor if you would like more explanation about this medicine.
Do not use Nimbex:
• if you are allergic to cisatracurium, any other muscle relaxant or any of the other ingredients in Nimbex (listed in section 6)
• you have reacted badly to an anaesthetic before.
Do not have Nimbex if any of the above apply to
you. If you are not sure, talk to your doctor, nurse
or pharmacist before you have Nimbex.
Take special care with Nimbex
Talk to your doctor, nurse or pharmacist before
having this medicine:
• if you have muscle weakness, tiredness or difficulty in co-ordinating your movements (myasthenia gravis)
• you have a neuromuscular disease, such as a muscle wasting disease, paralysis, motor neurone disease or cerebral palsy
• if you have a burn which requires medical treatment.
• you have ever had an allergic reaction to any muscle relaxant which was given as part of an operation
If you are not sure if any of the above apply to you, talk to your doctor, nurse or pharmacist before you are given Nimbex.
Other medicines and Nimbex
Tell your doctor if you are taking or have recently taken any other medicines. This includes any herbal products or medicines bought without a prescription.
In particular tell your doctor if you are taking any of the following:
• anaesthetics (used to reduce sensation and pain during surgical procedures)
• antibiotics (used to treat infections)
• medicines for uneven heart beats (anti-arrhythmics)
• medicines for high blood pressure
• water tablets (diuretics), such as furosemide
• medicines for inflammation of the joints, such as chloroquine or d-penicillamine
• steroids
• medicines for fits (epilepsy), such as phenytoin or carbamazepine
• medicines for mental illness, such as lithium or chlorpromazine (which can also be used for sickness)
• medicines containing magnesium
• drugs for Alzheimer's disease (anticholinesterases e.g. donepezil).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
If you are only staying in hospital for the day, your doctor will tell you how long to wait before leaving the hospital or driving a car. It can be dangerous to drive too soon after having an operation.
3 How Nimbex is given
How your injection is given
You will never be expected to give yourself this medicine. It will always be given to you by a person who is qualified to do so.
Nimbex can be given:
• as a single injection into your vein (intravenous bolus injection) .
Picture 2
Following discontinuation of infusion of Nimbex, spontaneous .
recovery from neuromuscular block proceeds at a rate comparable . . to that following administration of a single bolus.
Dosage in neonates (aged less than 1 month)
The use of Nimbex in neonates is not recommended as it has not been studied in this patient population.
Dosage in elderly patients
No dosing alterations are required in elderly patients. In these patients Nimbex has a similar pharmacodynamic profile to that observed in young adult patients but, as with other neuromuscular blocking agents, it may have a slightly slower onset.
Dosage in patients with renal impairment
No dosing alterations are required in patients with renal failure. In these patients Nimbex has a similar pharmacodynamic profile to that observed in patients with normal renal function but it may have a slightly slower onset.
Dosage in patients with hepatic impairment
No dosing alterations are required in patients with end-stage liver disease.
In these patients Nimbex has a similar pharmacodynamic profile to that observed in patients with normal hepatic function but it may have a slightly faster onset.
Dosage in patients with cardiovascular disease
When administered by rapid bolus injection (over 5 to 10 seconds) to adult patients with serious cardiovascular disease (New York Heart Association Class I-III) undergoing coronary artery bypass graft (CABG) surgery, Nimbex has not been associated with clinically significant cardiovascular effects at any dose studied (up to and including 0.4 mg/kg (8 x ED95)). However, there are limited data for doses above 0.3 mg/kg in this patient population. Nimbex has not been studied in children undergoing cardiac surgery.
Dosage in Intensive Care Unit (ICU) patients
Nimbex may be administered by bolus dose and/or infusion to adult patients in the ICU.
An initial infusion rate of Nimbex of 3 pjg/kg (body weight)/min
(0.18 mg/kg/hr) is recommended for adult ICU patients. There may
be wide interpatient variation in dosage requirements and these may
increase or decrease with time. In clinical studies the average infusion
rate was 3 pg/kg/min [range 0.5 to 10.2 pg/kg (body weight)/min
(0.03 to 0.6 mg/kg/hr )]. Table 6 provides guidelines for delivery of undiluted
Nimbex Forte (5 mg/ml) injection.
The median time to full spontaneous recovery following long-term (up to 6 days) infusion of Nimbex in ICU patients was approximately 50 minutes.
Table 6: Infusion Delivery Rate of Nimbex Forte injection 5 mg/ml
|
Patient (body weight) (kg) |
Dose (pg/kg/min) |
Infusion Rate | |||
|
1.0 |
1.5 |
2.0 |
3.0 | ||
|
70 |
0.8 |
1.2 |
1.7 |
2.5 |
ml/hr |
|
100 |
1.2 |
1.8 |
2.4 |
3.6 |
ml/hr |
The recovery profile after infusions of Nimbex to ICU patients is independent of duration of infusion.
Contraindications
Nimbex is contraindicated in patients known to be hypersensitive to cisatracurium, atracurium, or benzene sulfonic acid.
Special warnings and special precautions for use
Cisatracurium paralyses the respiratory muscles as well as other skeletal muscles but has no known effect on consciousness or pain threshold. Nimbex should be only administered by or under the supervision of anaesthetists or other clinicians who are familiar with the use and action of neuromuscular blocking agents. Facilities for tracheal intubation, and maintenance of pulmonary ventilation and adequate arterial oxygenation have to be available.
Great caution should be exercised when administering Nimbex to patients who have shown hypersensitivity to other neuromuscular blocking agents since a high rate of cross-sensitivity (greater than 50%) between neuromuscular blocking agents has been reported.
Cisatracurium does not have significant vagolytic or ganglion- blocking properties. Consequently, Nimbex has no clinically significant effect on heart rate and will not counteract the bradycardia produced by many anaesthetic agents or by vagal stimulation during surgery.
Patients with myasthenia gravis and other forms of neuromuscular disease have shown greatly increased sensitivity to non-depolarising blocking agents. An initial dose of not more than 0.02 mg/kg Nimbex is recommended in these patients.
Severe acid-base and/or serum electrolyte abnormalities may increase or decrease the sensitivity of patients to neuromuscular blocking agents.
There is no information on the use of Nimbex in neonates aged less than one month since it has not been studied in this patient population.
Cisatracurium has not been studied in patients with a history of malignant hyperthermia. Studies in malignant hyperthermia-susceptible pigs indicated that cisatracurium does not trigger this syndrome.
There have been no studies of cisatracurium in patients undergoing surgery with induced hypothermia (25 to 28°C). As with other neuromuscular blocking agents the rate of infusion required to maintain adequate surgical relaxation under these conditions may be expected to be significantly reduced.
Cisatracurium has not been studied in patients with burns; however, as with other non-depolarising neuromuscular blocking agents, the possibility of increased dosing requirements and shortened duration of action must be considered if Nimbex injection is administered to these patients.
Nimbex is hypotonic and must not be applied into the infusion line of a blood transfusion.
Intensive Care Unit (ICU) Patients
When administered to laboratory animals in high doses, laudanosine, a metabolite of cisatracurium and atracurium, has been associated with transient hypotension and in some species, cerebral excitatory effects. In the most sensitive animal species, these effects occurred at laudanosine plasma concentrations similar to those that have been observed in some ICU patients following prolonged infusion of atracurium.
Consistent with the decreased infusion rate requirements of cisatracurium, plasma laudanosine concentrations are approximately one third those following atracurium infusion.
There have been rare reports of seizures in ICU patients who have received atracurium and other agents. These patients usually had one or more medical conditions predisposing to seizures (e.g. cranial trauma, hypoxic encephalopathy, cerebral oedema, viral encephalitis, uraemia). A causal relationship to laudanosine has not been established.
Pharmaceutical particulars
Benzene sulfonic acid solution 32% w/v, Water for Injections.
Shelf life
Shelf life before dilution: 2 years.
Chemical and physical in-use stability has been demonstrated for at least 24 hours at 5°C and 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution has taken place in controlled and validated aseptic conditions.
Special precautions for storage
Store in a refrigerator (2 to 8°C). Do not freeze.
Store in the original package in order to protect from light.
For storage conditions of the diluted medicinal product, see section 5 of the Package Leaflet.
Instructions for use/handling
This product is for single use only. Use only clear and almost colourless up to slightly yellow/greenish yellow coloured solutions. The product should be visually inspected before use, and if the visual appearance has changed or if the container is damaged, the product must be discarded.
Diluted Nimbex is physically and chemically stable for at least 24 hours at 5°C and 25°C at concentrations between 0.1 and 2 mg/ml in the following infusion fluids, in either polyvinyl chloride or polypropylene containers.
Sodium Chloride (0.9% w/v) Intravenous Infusion.
Glucose (5% w/v) Intravenous Infusion.
Sodium Chloride (0.18% w/v) and Glucose (4% w/v) Intravenous Infusion. Sodium Chloride (0.45% w/v) and Glucose (2.5% w/v) Intravenous Infusion. However, since the product contains no antimicrobial preservative, dilution should be carried out immediately prior to use, or failing this be stored as directed under section 5 of the Package Leaflet.
Nimbex has been shown to be compatible with the following commonly used peri-operative drugs, when mixed in conditions simulating administration into a running intravenous infusion via a Y-site injection port: alfentanil hydrochloride, droperidol, fentanyl citrate, midazolam hydrochloride and sufentanil citrate. Where other drugs are administered through the same indwelling needle or cannula as Nimbex, it is recommended that each drug be flushed through with an adequate volume of a suitable intravenous fluid, e.g., Sodium Chloride Intravenous Infusion (0.9% w/v).
As with other drugs administered intravenously, when a small vein is selected as the injection site, Nimbex should be flushed through the vein with a suitable intravenous fluid, e.g., sodium chloride intravenous infusion (0.9% w/v).
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Nimbex 2 mg/ml solution for injection/infusion
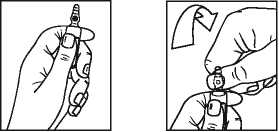
Instructions to open the ampoule (only applicable to 2 mg/ml ampoule) Ampoules are equipped with the OPC (One Point Cut) opening system and must be opened following the below instructions:
• Hold the bottom part of the ampoule with the hand as indicated in picture 1
• Put the other hand on the top of the ampoule positioning the thumb above the coloured point and press as indicated in picture 2
Picture 1
Information for the physician leaflet date: February 2014 Nimbex is a registered trademark of the GlaxoSmithKline group of companies
© 2014 GlaxoSmithKline group of companies. All rights reserved

GlaxoSmithKline

GlaxoSmithKline
• as a continuous infusion into your vein. This is where the drug is slowly given to you over a long period of time.
Your doctor will decide the way you are given the drug and the dose you will receive. It will depend on:
• your body weight
• the amount and duration of muscle relaxation required
• your expected response to the medicine. Children less than 1 month old should not have this medicine.
If you receive more Nimbex than you should
Nimbex will always be given under carefully controlled conditions. However, if you think that you have been given more than you should tell your doctor or nurse immediately.
If you have any further questions on the use of this medicine, ask your doctor.
4 Possible side effects
Like all medicines, Nimbex can cause side effects, although not everybody gets them.
If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet.
Allergic reactions (affects less than 1 in 10,000 people)
If you have an allergic reaction, tell your doctor or nurse straight away. The signs may include:
• sudden wheeziness, chest pain or chest tightness
• swelling of your eyelids, face, lips, mouth or tongue
• a lumpy skin rash or 'hives' anywhere on your body
• a collapse.
Talk to your doctor, nurse or pharmacist if you notice any of the following:
Common (affects less than 1 in 10 people)
• decrease in heart rate
• decrease in blood pressure.
Uncommon (affects less than 1 in 100 people)
• a rash or redness of your skin
• wheezing or coughing.
Very rare (affects less than 1 in 10,000 people)
• weak or aching muscles.
Reporting of side effects
If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5 How to store Nimbex
• Keep Nimbex out of the sight and reach of children.
• Do not use Nimbex after the expiry date shown on the pack after 'Exp'. The expiry date refers to the last day of that month.
• Store in a refrigerator (2°C - 8°C). Do not freeze.
• Store in the original package in order to protect from light.
• If diluted, store the infusion solution between 2°C and 8°C and use within 24 hours. Any unused infusion solution should be discarded 24 hours after it was prepared.
• Do not throw away any medicines via wastewater or household waste. Your doctor or nurse will throw away any medicine that is no longer required. This will help protect the environment.
6 Contents of the pack and other information,
What Nimbex contains
• The active substance is 2 mg/ml or 5 mg/ml cisatracurium (as besilate).
• The other ingredients are benzene sulfonic acid (32% w/v) and Water for Injections.
What Nimbex looks like and contents of the pack
Nimbex 2 mg/ml solution for injection/infusion comes:
• 2.5 ml clear glass ampoule in a box of
5 (each 2.5 ml ampoule contains 5 mg of cisatracurium)
• 5 ml clear glass ampoule in a box of 5 (each
5 ml ampoule contains 10 mg of cisatracurium)
• 10 ml clear glass ampoule in a box of
5 (each 10 ml ampoule contains 20 mg of cisatracurium)
• 25 ml clear glass ampoule in a box of
2 (each 25 ml ampoule contains 50 mg of cisatracurium)
Nimbex Forte 5 mg/ml solution for injection/ infusion comes in a box containing one 30 ml clear glass vial. Each 30 ml vial contains 150 mg of cisatracurium.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing Authorisation Holder:
The Wellcome Foundation Limited,
Stockley Park West, Uxbridge, Middlesex UB11 1BT Manufacturer: GlaxoSmithKline Manufacturing S.p.A., Strada Provinciale Asolana 90,
43056 San Polo di Torrile, Parma, Italy Other formats
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge:
0800 198 5000 (UK only)
Please be ready to give the following information:
Product name Nimbex 2 mg/ml
solution for injection Nimbex Forte 5 mg/ml solution for injection
Reference number 00003/0364 This is a service provided by the Royal National Institute of Blind People.
Leaflet date: February 2014 Nimbex is a registered trademark of the GlaxoSmithKline group of companies © 2014 GlaxoSmithKline group of companies.
All rights reserved
10000000122937
T1 Single twitch response as well as the first component of the Train-of-four response of the adductor pollicis muscle following supramaximal electrical stimulation of the ulnar nerve.
Enflurane or isoflurane anaesthesia may extend the clinically effective duration of an initial dose of Nimbex by as much as 15%.
Maintenance. Neuromuscular block can be extended with maintenance doses of Nimbex. A dose of 0.03 mg/kg (body weight) provides approximately 20 minutes of additional clinically effective neuromuscular block during opioid or propofol anaesthesia.
Consecutive maintenance doses do not result in progressive prolongation of effect.
Spontaneous Recovery. Once spontaneous recovery from neuromuscular block is underway, the rate is independent of the Nimbex dose administered. During opioid or propofol anaesthesia, the median times from 25 to 75% and from 5 to 95% recovery are approximately 13 and 30 minutes, respectively.
Reversal. Neuromuscular block following Nimbex administration is readily reversible with standard doses of anticholinesterase agents. The mean times from 25 to 75% recovery and to full clinical recovery (T4:T1 ratio > 0.7) are approximately 4 and 9 minutes respectively, following administration of the reversal agent at an average of 10% T1 recovery.